Bone And Soft Tissue Tumors
Primary malignancies of structural tissues of bone include a diverse group of tumors. Surgery is the mainstay of treatment. Occasionally amputation is necessary due to disease extent, or may permit better function with advanced orthotics. Chemotherapy has a demonstrated role in osteosarcomas with dramatically improved prognosis, but its effecacy has not been demonstrated in other primary bone malignancies. Radiation therapy for primary non-metastatic bone malignancies has an established role in the case of chordomas. Radiation is controversial in osteosarcomas and chondrosarcomas, but historic and modern data indicate that these tumors do respond to radiation.
Local control of osteosarcomas, chondrosarcomas, chordomas and giant cell tumors requires excellent surgery with radiation therapy an adjunctive measure in cases of microscopically positive margins for malignancy. Radiation is also used as a priamry treatment for surgically inaccessible locations such as the axial skeleton and in palliation for massive or metastatic sarcomas which are inoperable. Radiotherapy is a major treatment in symptom palliation of bone metastases.
Natural History
Osteosarcoma
Osteosarcoma is the most common primary malignant bone tumor in children and young adults. The median age is 20 years for all osteosarcoma patients. Classic osteosarcoma is nearly 80% and is always a high grade spindle cell tumor which produces osteoid (immature) bone. The most frequent sites are the metaphyseal areas of the distal femur or proximal tibia which are the steis of maximum bone growth.
Peraosteal osteosarcoma is a juxtacortical osteosarcoma variant that tends to occur mostly in the posterior distal femur. Paraosteal tumors tend to metastasize later than classical osteosarcoma and are low grade.
Periosteal osteosarcoma is a juxtacortical osteosarcoma variant that most often involves the femur followed by the tibia and are intermediate in severity between classical and pera-osteal sarcomas.
Patients with Paget's disease (disordered bone growth), retinoblastoma or prior radiation are at increased risk of developing osteosarcoma.
Pain, swelling are frequent early symptoms with pain usually intermittant at first then progressively worsening. Osteosarcoma spreads hematogenously, favoring lungs as the most common metastatic site.
Prognostic factors include tumor site (distal lower extremity most favorable), size, presence and location of mets (solitary/oligo lung mets are resectable and have a survival rate approaching M0 disease). Histologic response to chemotherapy and complete resection with negative margins are significant prognostic factors for patients with osteosarcoma of the extremity and trunk.
Adverse prognostic indicators (COSG - Cooperative Osteosarcoma Study Group) include axial tumor site, male sex and long history of symptoms were associated with poor response to chemotherapy. Patient age, primary tumor site, primary metastases had significant infulence on outcome at diagnosis. For extremity sites, tumor size and location within the extremity were prognostic. Elevated LDH was associated with worse outcomes.
Response to chemotherapy and surgical success were identified as the key prognostic predictors. For osteosarcoma with initially diagnosed metastatic disease, the number of sites, and completeness of surgical resection of all clinical detected metastases were independant prognostic factors.
Clinical Bytes
- Prevalence: Osteosarcoma > chondrosarcoma > Ewing's Sarcoma > MFH (malignant fibrous histiocytoma
- Age: 60% occur between 10 and 20 years which is the age of most active skeletal growth
- 80% are in the long bones until epiphysial (growth plate) closure; then apendicular skeleton predominates
- In older patients (age > 60 years) > 50% arise from other conditions such as Paget's Disease or fibrous dysplasia
- Osteosarcoma: Malignant osteoid is the hallmark which is not seen in chondrosarcoma.
- Most common bone tumor in children
- 75% present in the metaphyses of the long bones (transition zone between shaft and growth plate) with local pain and swelling
- 85% are high grade
- Osteosarcoma arising from radiation therapy or chemotherapy does not have a worse prognosis.
- Osteosarcomas are associated with LiFraumeni (p53) and retinoblastoma
- Most common in femur > tibia > humerus
- Most common distant metastases is in the lung (recall the Cade Technique experiment)
- Periosteal (juxtacortical) osteosarcomas are usually low-grade localized disease with rare distant metastases.
- Most periosteal osteosarcomas present in the popliteal fossa
- 80 - 90% are curable with surgery alone.
- Chondrosarcomas
- ~25% of all bone tumors
- Most common in femur with frequent local recurrence
- Distant metastases are less common than osteosarcoma
- 1/3 are high grade
- MFH: locally very agrressive with frequent distant metastases. Often presents with pathologic fracture.
- Fibrosarcoma: high grade, behaves like osteosarcoma. Often presents with pathologic fracture.
- Chordoma:
- Physaliferous cell ("bubbly cells")
- Most often found in the sacral-coccygyl area, base of skull or spine.
- Clinical presentation is specific to site of disease
- Giant cell tumors are multinucleated osteoclasts which are mostly benign. Cyst formation, hemorrhage and necrosis are important to radiation sensitivity. They recur frequently locally (45 - 60%)
- Lung mets are common in osteosarcoma, chondrosarcoma, MFH.
| Ewing's Sarcoma | Osteosarcoma |
|---|---|
| Lytic, destructive lesion | Sclerotic lesion |
| Diaphysis (shaft) | Metaphysis |
| Onion Skin radiograph | Sunburst pattern radiograph |
Radiologic Imaging and Work up
Plain film x-rays will provide a highly reliable diagnosis. Age, location of tumor in bone (epiphysis, metaphysis or diaphysis and axial v. appendicular skeletal site aids in the determination of a radiologic diagnosis.
Osteosarcomas arise in the metaphyseal region of the long bones and may extend into the diaphyseal or epiphyseal region or both of the affected bone. Cortical bone is destroyed and a periosteal reaction gives rise to the Codman's Triangle and periosteal spicules. A soft tissue mass is usually present and can be seen on MRI. MRI can also image intermedulary skip metastases.

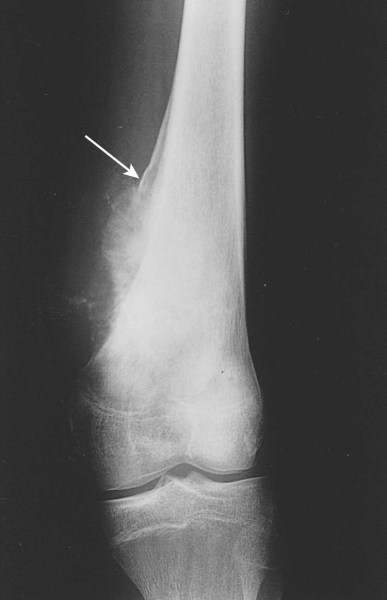 |
Staging AJCC 7th Ed.
| T1 | Tumor ≤ 8 cm |
| T2 | Tumor > 8 cm |
| T3 | Discontinuous tumor in the primary bone |
| N0 | No nodes |
| N1 | Node positive |
| M0 | No distant mets |
| M1a | Lung mets only |
| M1b | Other distant sites |
Stage Grouping
| Grade | T1 | T2 | T3 | N1 | M1a | M1b |
|---|---|---|---|---|---|---|
| G1 | IA | IB | IB | IVB | IVA | IVB |
| G2 | IA | IB | IB | IVB | IVA | IVB |
| G3 | IIA | IIB | III | IVB | IVA | IVB |
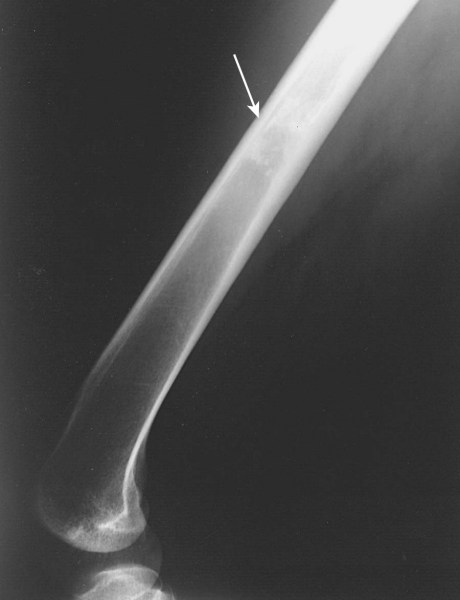
Chondrosarcoma: Benign cartilaginous lesions (enchondroma or osteochondroma) may have subtle differences from chondrosarcomas. Enchondroma induces endosteal scalloping and gradual cortical remodelling. Evolution into cortical destruction indicates malignancy. These should be monitored with serial radiographs.

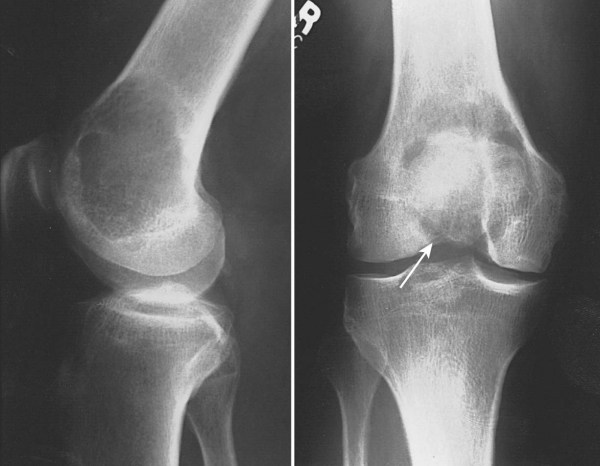
Giant Cell Tumors: These tumors are metaphyseal lytic tumores. They usually cause cortical thinning. Bone repare may not be radiographically demonstrated for up to 2 years after radiation. Serial studies are essential to avoid confusion with tumor progression or involution of the sclerotic rim.

Pathology
Chondrosarcoma appear low grade and similar to benign cartilaginous growths. Extensive sampling is necessary. Malignant osteod is not seen.

Osteocarcoma Osteoid is the hallmark of osteosarcoma. The presence of other malignant mesenchymal tissues within osteosarcomas suggest the precursor cell is more primitive mesenchymal pluripotential cell.
Chordoma is characterized by "bubbly cells" with intracytoplasmic mucoid droplets. They arise from the notochord in the axial skeleton and ordinarily during development undergo gradual obliteration within the vertebral bodies. The central portion of the intervertebral disk may persist.

Radiotherapy
Osteosarcoma
Malignant primary bone tumors are reare, represnting less than 1% of all malignancies. Osteosarcoma is the most common of these uncommon malignancies. It is twice as common as chondrosarcoma and three times as common as Ewing's Sarcoma, and ten times as common as MFH of the bone. Osteosarcomas are bimodal in incidence with 60% between 10 - 20 years, and 10% in patients older than 60 years. Males predominate slightly at 1.3:1 and girls tend to peak at a younger age than mailes due to the differences in developmental physiology (17 y.o. v. 18 y.o. in males). Distal femur sites are more than 50% of adolescent cases. Until epiphyseal closure, long bones are nealry 80% of cases. After skeletal maturity, the incidence equalizes between long bones and axial skeleton. Then skull and axial bones represent 40% of the cases. Radiaiton has been implemented in development of osteosarcomas. 222RA was used for the treatment of bone TB or ankylosing spondylitis were found to have a 200x increased risk for bone sarcoma development at doses estimated to be around 4.2 Gy. Bone sarcomas were the most common secondary malignancies reported with doses reported < 10 Gy to 80 Gy. Most of the secondayr sarcomas were well away from the central axis in 50% were in the penumbra, with an addional 30% in superficial tissues superficial to dmax.
80% of osteosarcoma patients develop lung metastases within 2 years of diagnosis. Amputation is inadequate treatment for the majority of patients. The Cade technique was developed with a goal to spare children destined to develop lung metastases from amputation. He treated the primary tumor at diagnosis to 70 - 90 Gy in 8 - 12 weeks. Patients free of systemic disease at 6 - 9 months underwent amputation. Many were found to have no clinical evidence of disease in the amputation specimens. Later in 1973, Stevens reported 10 patiens with local oseosarcoma traed with 79 - 100 Gy with 60Co or 2 MV x-rays. No evidence of tumor was found in 6 of 7 surgical specimens. 6/10 patients were free of disease at 30 months follow up. This information suggested that pre-operative radiation resulted in reduced likelihood of systemic metastases.
MSKCC in 1975 proposed high dose MTX, vincristine, adriamycin and cyclophosphamide as adjuvant therapy after surgical resection of primary osteosarcoma. There was a clear benefit to chemotherapy in the post operative setting. Due the success of this regimen, MSKCC proposed pre-operative chemotherapy with response used as a guide for post-operative chemotherapy. They found better limb preservation with complete removal of tumor. Neoadjuvant chemotherapy is the present standard of care in osteosarcoma management with surgical resection after 2-3 months of neoadjuvant chemotherapy followed by post-operative adjuvant chemotherapy for an additional 4 - 6 months.
Radiation therapy for osteosarcoma was largely supplanted by neoadjuvant and adjuvant chemotherapy. However, a German/Austrian study (Cooperative German/Austrian Osteosarcoma Study Group) reviewed 1982 consecutive patients entered prospectively onto neoadjuvant chemothearpy studies between 1979 and 1998. 67 patients had non-metastatic, high grade primary tumors of the pelvis, excluding sacral primaries. 11 patients received RT in some form to achieve local control. Compared with a similar group of unirradiated patients, the OS5 was 29% in the irradiation group and 0% in the unirradiated group. These authors conclude taht unresectable osteosarcomas or those with inadquate margins should be treated with radiation therapy as part of the regimen.
Stea contends that small cell varant of osteosarcoma may be as radiosensitive as Ewing's Sarcoma. Tumors in teh sacral and para-acetabular regions may benefit from pre-operative, intraoperative or post-operative radiation therapy, either alone or in combination with other therapies. Russian studies reported that EBRT to a dose of 60 Gy (40 - 68 Gy) after neoadjuvant chemotherapy in 31 who refused amputation for extremity osteosarcomas found OS, PFS, DM-free survivals at 5 years were 61%, 56% and 61%. Survival was far higher in those who had a significant response to chemotherapy at 91% v. 35%. Radiotherapy is an option for those who have an excellent response to chemotherapy. Two recent studies indicate that postoperative radiotherapy improves results for patients with positive margins.
Technique Radiotherapy ot unresectable osteosarcoma requires a high dose and limited volumes as well as concomitant chemotherapy using IMRT or 3D-CRT. At least 75 Gy should be the goal without chemotherapy.
Chondrosarcoma
Chondrosarcoma is the second most common malignancy of the bones. They are reare in age younger than 20. The risk goes up after age 20 until around age 75. Chondrosarcomas can develkp anywhere there is cartilage. MOst are found in the bones of the pelvis, thigh or humerous. A SEER analysis revewled 2890 cases were reported between 1973 and 2003. Only grade and stage predict survival.
Radiation therapy is indicated as a primary treatment when definitive surgery is not possible and post-operatively when surgical margins were inadequate. PMH reviewed 23 years of chondrosarcoma data. 50% were controlled locally following treatment, 25% were disease free at 15 years. Doses were 50 - 55 Gy at 2.5 Gy/fraction. THead and Neck and truncal tumors hae an 85% local recurrence rate with surgery alone, thus radiation is useful where surgical resection is inadequate.
MSKCC demonstrated that the mesenchymal variant of chondrosarcoma (in 35 patients) with predominant hemangiopericytomatoid variant and those with a small cell undifferentiated pattern had responsveness to both chemotherapy and radiation. Low grade base of skull chondrosarcomas have been treated with proton RT with local control at 5 years at 82%. Dose was 67 CGE.
Conventional chondrosarcomas have no established chemotherapy regimens. Dedifferentiated chondrosarcomas could be treated similarly to osteosarcoma but there is not a clear consensus but no major disagreement. Likewise, mesenchymal chondrosarcomas could be treated per Ewing's, but again there is not a clear consensus.
Chordoma
50% of chordomas arise in the sacrum, 35% in the clius and 15% in the vertebrae. 5-43% eventually metastasize, primarily to the lungs. Prolong survival with metastatic disease is not uncommon. Mallinckrodt noted that no patient was controlled and the group treated with conventional radiation alone did worst with no survivors. Lumbosacral tumors treated with surgery plus radiaiton had longer mean disease freee survival (6.6 years) than surgery alone (4.1 years). They recommend doses of 55 - 60 Gy at 2 Gy/fraction.
For base of skull chordomas, treatment to 0.1 CGE with proton/photon mixed beam demonstrated local control was a highly significant predictor of DFS. OS following local relapse was poor at 5% (5 year, zero at 7 years). Complete surgical resection with negative margins can be curative, but this rarely can be achieved. Poor long term survival after local recurrence demonstrates a strong need for a joint surgical/radiation approach.
Surgical excision must be en bloc, with poor local control wehn the tumor is violated during resection and a second radical resection will rarely achieve local control. If positive margins or gross residual disease remains after primary surgery, post-operative RT is needed. A French report on 100 patients treated with mixed photon/proton beams for BoS and c-spine demonstarted local control rates at 2 and 4 years were 86% and 54%. Minimum dose and dose homogeneity were important outcome related factors. Median total dose was 67 CGE. Residual tumor > 30 cm3 was a poor prognostic factor.
Giant Cell Tumor
Giant cell tumors are more prevalent in femails, and rarely occur before final skeltal maturity. Most occur between 20 and 40 years. The majority are surgically managed with local control rates of 85 - 90%
Radiation therapy at the Mayo clinic in the middle 1/3 of the 20th Century demonstrated local failure in patients treated with surgery or surgery followed by radiation therapy to be identical at 43%. GCT tumors are quite radioresponsive. MDACC, Mayo and UFL have series treated with radiation for gross unresectable disease to doses of between 36 and 66 Gy depending on surgical extent. Prolonged local control of gross residual disease with RT in teh dose range of 40 - 50 Gy has been demonstrated. Radiaiton is indicated for unresectable leasions in the axial skeleton and after subtotal resection at any site. Radiation may be appropriate if there are microscopically positive margins. Conformal therapy or IMRT is prefered to limit dose to normal bone and organs.
.
Staging and Clinical Workup
- H&P
- lab studies including ESR, alk phos
- Plain x-rays and CXR or CT chest: Codman's triangle, periosteal bone spicules.
- Primary tumor often seen as cloud-like density
- CT/MRI of primary areas and chest to evaluate soft tissue extensions
- Bone scan to detect skip mets and consider PET
- Biopsy after complete radiologic evaluation, taking care that incision will be within radiation/surgical field. Biopsy should be performed at the institution where treatment will occur.
Treatment Recommendations
Generally limb sparing strategies are preferred. This may involve a combination of neoadjuvant chemotherapy, radiation therapy and surgery. Input from an orthopedic oncologist is essential in determining whether limb sparing is possible or desirable. Final limb function may be better with prosthesis than with partially resected and/or irradiated limbs. Pediatric radiation has added implications on bone growth before closure of the epiphyseal plates, which may seriously adversely affect function.
Osteosarcoma
Per the NCCN guidelines for bone cancers, Osteosarcoma workup is as follows:
- Plain films with abnormal radiograph suspicious for ostesarcoma (Codman's sign in the metaphyseal region of th =e long bones with periosteal spiculations call for referral and biopsy at a center which will treat the osteosarcoma
- MRI of the lesion is indicated to look for a soft tissue mass
- Chest imaging should be included as lung metastases are common and can be surgically excised.
- PET/CT scan and/or bone scan is recommended
- LDH and alk phosphatase levels
Treatment recommendations for low grade osteosarcoma both intramedullary and on the surface consist of wide local excision and if function cannot be preserved, amputation and prosthesis. For periosteal osteosarcoma, consider chemotherapy prior to WLE.
- High Grade Disease (Post-WLE): Chemotherapy consisting of multiagent IV/IA infusion with growth factor support
- CDDP+adriamycin
- MAP (mtx, CDDP, adriamycin
- adriamycin, CDDP, ifosfamide, high dose MTX
- Ifosfamide/etoposide
- ifosfamide/CDDP/epirubicin
- Low Grade Disease: Surveillance
For high grade disease, treat with pre-operative chemotherapy with above multiagent chemotherapies. Reassess and restage the tumors including chest imaging to assess M1a status (chest mets alone). Consider PET and bone scans. If the tumor is unresectable, follow with RT ± sensitizers or chemotherapy as above. If a resection is possible, then wide local excision is desirable. If margins are negative, and a good response to chemotherapy by pathologic mapping is demonstrated.then additional post-operative chemotherapy is recommended. If there is a poor response consider secondary chemotherapies:
- Pre-operative chemotherapy → surgery → post-operative chemotherapy for 4 - 6 months.
- Consider clinical trials.
- Radiotherapy for inoperable disease or close/positive margins: 60 - 75 Gy with shrinking fields.
- For pelvic tumors Consider intra-arterial chemotherapy (CDDP/doxorubicin) + RT 60 - 70 Gy
- If possible in M1a disease, resect lung metastases which improves survival.
Second line chemotherapies:
- Docetaxel + gemcitabine
- cyclophosphamide + etoposide
- cyclophosphamide + topotecan
- gemcitabine alone
- ifosfamide + etoposide
- ifosfamide, carboplatin, etoposide
- high dose MTX, etoposide and ifosfamide
- 153-Sm-EDTMP for relapsed or refractory disease beyond second line chemotherapy
For high grade disease with positive margins and good response to chemotherapy, consider additional chemotherapies as described above, or additional local therapy. With poor response by pathologic mapping, additional local therapy and alternative chemotherapies.
Surveillance includes periodic chest imaging, laboratory studies, PET/Bone scans (category 2b) and repetative functional reassessments. Follow up Q3M for years 1 and 2, then Q4M for years 3 and 4, then Q6M for years 4 and 5.
Relapsed osteosarcoma is treated with chemotherapy as described above and followed for response. If there is no response, then further resection, if possible, or SamariumEDTMP, palliative RT, clinical trial or best supportive care is indicated.
MFH
Malignant fibrous histiocytomas are treated as osteosarcomas.
Chondrosarcomas: Low Grade and Intracompartmental
Primary Treatment
Primary treatment of low grade and intracompartmental chondrosarcomas consists of surgical wide local excision if completely resectable with widely negative margins or amputation if necessary.Otherwise, if unresectable, the radiation therapy should be considered. This should be followed by PE, chest and lesion imaging every 6-12 months for 2 years then annually.
For local recurrences, a repeat wide local excision is indicated if resectable. If not, then radiation therapy should be considered. In the event surgery is unable to achieve negative margins, then adjuvant radiation therapy should be considered.
There are no known standard chemotherapy regiments for chondrosarcomas. For mesenchymal chondrosarcomas follow the recommendations for Ewings Sarcomas. For de-dedifferentiated chondrosarcomas follow the recommendations for osteosarcomas.
Surgery is the primary treatment (WLE or amputation). Radiation therapy is used for inoperatble tumors and close or positive margins. Radiation doses for chondrosarcomas are 60 - 70 Gy EBRT and 15 - 30 Gy IORT.
Giant Cell Tumors
Surgical resection with meticulous currettage or complete resection gives LR rates as low as 10-20% but historically has been around 45-60%. Post-opeartive RT for inoperable tumors, positive margins and for situations where surgery would create significant functional disability. Radiation doses are 40 - 55 Gy.
Chordoma:
Surgery followed by Radiation therapy or radiation therapy alone for inoperable tumors to 66 - 70 Gy. Consider SRS or proton/charged particle therapy if available.
Aneurismal bone cyst
Surgery or radiation to 25-30 Gy for recurrent disease and surgically inaccessable (ie vertebral disease).
Radiation Toxicity and Dose Limitations
- > 20 Gy causes premature epiphysis closure
- > 40 Gy ablates bone marrow
- ≥ 50 Gy to the cortex significantly increases fracture risk
Radiation can cause abnormal bone and soft tissue grown and development, permanently weakening the affected bones., causing scoliosis, fibrosis resulting in decreased range of motion, or joint stiffening. Radiation induced vascular changes increase infection susceptibility risk of fracture, lymphedema, and osteoradionecrosis.
Intensive rehabilitation and physical therapy is vitally important to preservation of limb function, especially for younger/pediatric patients.
Studies: Osteosarcoma
- Eilber, 1986:
- Randomized studies have established the role of neoadjuvant and adjuvant chemotherapy in patients with localized, resectable osteosarcomas, in the prevention of relapse/recurrence.
- Cooperative German/Austrian Osteosarcoma Study Group, Ozaki 1983
- A subset analysis of 67 patients with high grade pelvic osteosarcomas, M0
- Radiation therapy improved survival for patients with intralesional excision and unresectable tumors.
- Machak, 2003:
- 31 Patients with non-metastatic osteosarcoma who refused surgery
- Treated with induction chemotherapy → radiation therapy to a median dose of 60 Gy.
- OS5: 61%, PFS5: 56%, DMFS5: 62%.
- Good responders ahd OS5 of 90% and DMFS of 91%
- Non responders had PFS of 31% at 3 years and 0% at 5 years.
Studies: General/Mixed Preoperative
- Wagner, 2009 Preoperative RT
- 48 patients with solid bone tumors
- 52% chordomas
- 31% chondrosarcomas
- 8% osteosarcomas
- 4% Ewing's Sarcoma
- Pre-operative RT to 20 Gy followed by resection followed by post-operative RT to a total dose of 50.4 Gy
- 5 year OS: 65%; DFS: 53.8%; LC 72%. No difference was seen between histopathologies
- This approach appears to inhibit tumor seeding and allows for dose escalation without hgih dose pre-op RT or large field post-op RT
- 48 patients with solid bone tumors
Chordoma
Several studies have indicated charged particle treatment and/or radiosurgery may improve local control.
Common Radiation Techniques
- Treat entire surgical bed + 2 cm margins and include surgical scar in field
- Bolus scar for the first 50 Gy
- Treatment Planning with CT/MRI GTV identification
- Exclude skin over anterior tibia if possible
- Initiate physical therapy early in treatment to improve functional outcome.