PEDS: Rhabdomyosarcomas
Pediatric rhabdomyosarcoma is a highly malignant sarcoma arising from embryonal mesenchyme with the potential for differentiating into striated muscle. It was first described in 1854 and characterized into four classic forms in 1958,
- Spindle cell and botryoid
- Classic embryonal
- Alveolar
- Undifferentiated
Rhabdomyosarcoma can arise anywhere in the body , is rapidly invasive and disseminates early. Initially surgery was the only treatment which was highly disfiguring and not available to those who had disseminated disease. Early in the 20th century, high dose radiation therapy was added and increased the potential for local control but it came with its own set of morbidities.
Advances in chemotherapy throughout the 20th century increased control of distant metastases and improved local control, but surgery and radiation continue to play important roles in the treatment of rhabdomyosarcoma. Chemotherapy reduced the need for aggressive survery and large volume radiation therapy. Overall survival rates have increased form 15% - 25% to more than 70%.
The tumor itself is rare with clinical and biological heterogeneity. There have been a number of study groups over the years which have had clinical studies leading to the substantial improvoments in outcomes. The Intergroup Rhabdomyosarcoma groups did three studies:
- IRS-I: 1972-1978 OS 55%
- IRS-II 1978 - 1984 OS 63% (Designed to improve outcomes in subgroups with poor outcomes)
- IRS-III 1984 - 1991 OS 71%
- IRS-IV 1991 - 1997 OS 86% (non-mets) 39% (mets) Based on more biolgically oriented staging systems (IV and V)
- IRS-V 1997 - 2005/6
There is now a database of 4000 children with rhabdomyosarcoma treated on these studies. the COG has assume responsibility for these studies. In Europe, SIOP and UK CSTG are parallel organizations.
Epidemiology and Demographics
RMS is the most common soft tissue sarcoma of childhood representing almost half of the sarcomas in children. 70% occur before the age of 10 and there is a slight male predominance. The peak incidence is 2-5 years. Congenital abnormalities have been identified in as many as 1/3 mostly GI, GU cardiovascular and CNS. The incidence is 4.4 / 1M white and 1.3/1M black.
The majority of cases are sporadic, but a small number are associated with several genetic syndromes:
- Li Fraumeni (p53) Syndrome
- Neurofibromatosis I
- Costello Syndrome (HRAS Gene)
- Noonan Syndrome
- Beckwith-Wiedemann Syndrome
There are postulates that in-utero exposure to marijuana, cocaine use and radiation may have a role in the development of RMS.
Embryonal and alveolar RMS appear appear to differ in genetics.
Embryonal RMS is often characterized by a loss of heterozygosity of 11p15.5 suggesting the presence of a tumor suppressor gene. In addition gene amplification is rare in embryonal RMS, with whole chromosome gains (Ch 2,8, 12, 13) being common and hyperdiploidy also common.
In contrast, alveolar RMS has common gene amplification and is associated with t(2:13) translocation in 70% and t(1:13) in 20%. There genes are the PAX3 or PAX7 with FHKR.
Mesenchymal fibroblast cells differentiate inot skeletal muscle under the control of the MyoD protein family (actin, myosin, dsmin, myoglobin).
In alveolar RMS, n-myc amplification predicts a fatal outcome. N-myc amplification is uniformly absent in embryonal RMS.
p53 is a G1 checkpoint. Normal p53 in conjunction withmyc drives damaged cells at the G1 checkpoint toward apoptosis.
RMS histologies by prognosis from best to worst:
| Histology | OS5 |
|---|---|
| botryoid | 95% |
| spindle cell | 88% |
| embryonal | 66% |
| alveolar | 54% |
| undifferentiated | 40% |
- Spindle cell and botryoid
- classical embryonal
- Alveolar
- Undifferentiated
RMS is divided into favorable and unfavorable sites. These favorable sites have a 94% OS3. The favorable sites are:
- Orbit
- Non-parameningeal Head and Neck
- Non-prostate/bladder Genitourinary sites
- Biliary
The non-parameningeal head and neck sites are:
- Scalp
- Cheek
- Parotid
- Oral Cavity
- oropharynx
- larynx
For unfavorable sites, the overall 3 year survival rate is 70%.
The para-meningeal head and neck sites are:
- Middle ear
- Mastoid
- Nasal Cavity
- Nasopharynx
- Infratemporal Fossa
- Pterygopalatine Fossa
- Paranasal sinuses
- Parapharyngial spaces
There are a number of sites associated with a high propensity for lymph node metastases. Disease occurences at these sites require lymph node dissection. The following sites are associated with a 20% Lymph node metastases rate:
- Paratesticular in patients > 10 years old
- Bladder: (pelvic lymph nodes)
- Head and Neck: nasopharynx (LND not typically done for NP tumors)
- Extremities: Upper -- axillary LN, Lower: inguinal/femoral LN
The IRS-IV study called for routine sampling of LN in the extremity due to a 10% cN+ and a 50% rate of pN+ disease. Of those who were clinically node negative, 17% were pN+.
Non-regional lymph nodes by site are:
- Upper Extremity: Scaline Nodes
- Pelvic (paratesticular, vagina, uterus): Inguinal
- Retroperitoneal: para-aortic unless immediately adjacent to lesion
- Lower Extremity: iliacs, PALN
Clinical Presentation
RMS can occur in any anatomic site with the presence of skeletal muscle and several additional sites:
- bladder
- prostate
- vagina
- uterus
- urethra
- paratesticular region
RMS commonly presents a mass with poorly defined margins with symptoms related to the primary site: GU may present with obstructions or the mass may protrude from the vagina (botryoid) or cervix or urethra. Grape-like clusters are called botryoid sarcoma. Prostate and bladder RMS may also appear botryoid and may protrude into the bladder lumen. Hematuria, urinary frequency or retention may occur. Less than
Paratesticular RMS presents with a mass and may be mistaken for hydrocele, incarcerated hernia, or testicular torsion. RMS in the extremities may be a palpable mass or cause pain or limit function.
Paratesticular RMS arises from the distal spermatic duct.
Parameningeal sites may present with upper airway obstruction or a palpable mass. It can erode into the base of skull and cause CN palsies. If it penetrates the skull, it can impinge on the brain and cause mass effect with headache, vomiting and diploplia.
Non-parameningeal RMS can cause a palpable mass on the cheek or aerodigestive tract obstruction. Orbital RMS usually presents with proptosis, discoloration or limited extraocular motions. In the trunk, RMS primarily resents as a mass effect.
Diagnostic Evaluation
The workup including H&P should focus on the extent of local disease and the presence of metastases. This is particularly true for those sites that have increased propensity for LN metastases (> 20&) including extremities, nasopharynx, bladder, and paratesticular for age > 10. RMS extends focally and along a fascial plane and into surrounding tissues and may have indistinct margins.
Imaging
Imaging workup includes CT, MRI and plain films. Genitourinary RMS is often initially investigated with ultrasound, voiding cystourethrogram and barium enema, along with cystoscopy or pelvice EUA. CT and surgery are used to evaluate the draining lymphatics of the extremities and GU sites.
Chest CT is optimal for evaluating lung metastases as the most common site of distant metastases. Bone scans may be used to detect bony metastases, but cannot be relied upon for base of skull involvement.
Parameningeal tumors should be imaged with MRI. MRI is also used to determine the spinal cord patency when cord related symptoms are present.
PET/CT is being used with increasing frequency for detecting regional lymph node metastases and distant metastases.
Biopsy
For parameningeal sites, specific additional work up is required: CSF cytology are used to determine if a tumor is parameningeal, and if positive, a neuro-axial MRI should be obtained.
Staging
The AJCC staging is straightforward, but there are multiple staging systems in use. The International Rhabdomyosarcoma Study group (IRS-III) developed a pre-treatment staging system based on tumor, node and metastases, similar to a SIOP staging system. IRS-IV and IRS-V and COG trials now use this TNM staging system.
| Stage | Site | Size | Nodes | Mets |
|---|---|---|---|---|
| I | favorable | N0 or N1 | M0 | |
| II | unfav | < 5 cm | N0 | Mo |
| III | unfav | >5 cm or N1 | M0 | |
| IV | M1 |
| T1a | Confined to antatomic site of origin ≤ 5 cm |
| T1b | > 5 cm |
| T2a | extension to or fixed to adjacent tissue ≤ 5 cm |
| T2b | adjacent tissue involvement > 5 cm |
| N1 | Regional LN involvment |
| M1 | Distant mets |
The pre-op staging criteria for RMS
| Stage | Sites | Invasiveness | Size | Nodes | Mets |
|---|---|---|---|---|---|
| 1 | FAVORABLE:
|
T1 or T2 | Any size | Any N | M0 |
| 2 | UNFAVORABLE
|
T1 or T2 | < 5 cm | N0/Nx | M0 |
| 3 | UNFAVORABLE | T1 or T2 | < 5 cm | N1 | M0 |
| UNFAVORABLE | T1 or T2 | > 5 cm | N0 | M0 | |
| 4 | All sites | T1 or T2 | Any size | Any N | M1 |
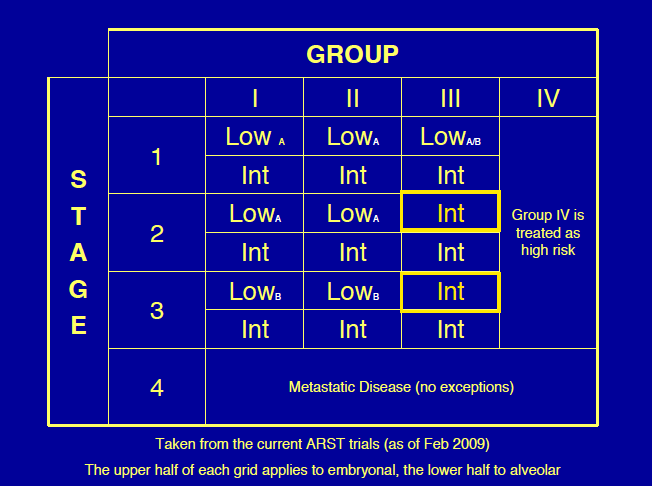
Note: Although tumor invasiveness is included, it does not appear to discriminate in the determination of pre-op stage grouping. IRS-IV, IRS_V and COG trials are using this staging system which incorporates these factors. Stage 1 tumors are in favorable sites, All other stages are unfavorable sites. Stage 2 tumors are small (<5 cm), and are N0, Stage 3 tumors are large or with N+ disease. Stage 4 is distant metastases. This staging system has been validated for outcomes.
There is also an IRS Grouping System which segregates patients into prognostic groups. This grouping system describes the post-surgical disease extent at the time. This group reflects absence of disease, microscopic disease, gross disease or metastatic disease.
| Group | Description | Patient Percentage |
|---|---|---|
| I | Localized Disease completely resected
|
13% |
| II | Gross Total Resection
|
20% |
| III | Subtotal Resection
|
48% |
| IV | Metastatic Disease | 18% |
Most RMS patients present with Group III disease. Group III is essentially an R2 resection or biopsy only. Only 25% of Clinical Group III patients achieve a gross total resection.
The IRS has developed a 3 tier risk grouping as part of the IRS - IV risk group. These groups are segregated as follows:
- Low Risk (OS5 90% - 95%) non-metastatic embryonal and
- favorable site all groups or
- unfavorable site groupts I/II (R0/R1 resection)
- Intermediate Risk:
- non-metastatic Group III (R2 resection), embryonal histology, any site. (OS5 70-85%)
- non-metastatic unfavorable histology and any site. (OS5 5-60%
- High Risk includes all metastatic disease: < 10 y.o. embryonal, (OS5 25%-35%)
The primary disease site determines outcomes. The data from IRS-II and IRS-III demonstrate disease site, in part, determines the maximum feasible extent of surgical resection which determines the IRS grouping. Completeness of surgical resection is also determined by the degree of invasiveness. Most orbital lesions are Group III (R2) at around 73.5% as are parameningeal lesions.
Most bladder and prostate lesions are also Group III. Non-bladder/non-prostate GU lesions and most extremity tumors are Group I (R0) or Group II (R1). or are metastatic at diagnosis (Group IV)
Primary site also determines the propensity for lymphatic spread. GU, abdominal, pelvic, and extremity disease commonly involves lymph nodes (≥ 20%). Tumors of the head and neck, trunk and female genitalia rarely involve nodes.
Prognostic Groups
Treatment
Current COG studies stratify into one of 3 risk groups described above. Treatments for RMS in the IRS studies varies based on these risk groups. The general treatment paradigm is: Maximum safe resection or biopsy alone → chemotherapy ± radiation with the timing of CRT based on the risk groups.
Prognostic factors include age with age > 10 being worse (EFS 14% than age younger than 10 (EFS 47%) Other factors portending higher risk include subarachnoid space involvemnt with skull base erosion in parameningeal head and neck disease, CN palsy and intracranial extension. DFS is reduced to 51% from 81% without risk factors.
Chemotherapy agents generally used are VAC (vincristine, actinomycin D and cytoxan. Isfosphamide is also used in some subsets of RMS.
International RMS Studies
The sentinel trial that supported the use of chemotherapy in RMS dates to the 1970's. Hen reported on a study comparing vincristine/actinomycin D with observation after surgery. This study demonstrated an advantage in post-operative use of chemotherapy.
IRS-I Study This study demonstrated that for
- Group I/Favorable histology, radiation therapy was not needed.
- Group II: Radiation therapy + Vincristine and Actinomycin D but no need for Cytoxan
- Group III-IV: RT + VAC x 2 years (no adriamycin needed. Distant metastastes failure more common than local failure
- No dose response for RT and no difference with large v. small field radiation therapy.
- Parameningeal RMS had increased CNS relapse if certain high risk features were present.
IRS-II This study determined that for:
- Group I (R0) VA x 1 year gave the same results as VAC x 2 years except in unfavorable histologies
- Group II (R1): RT + VA x 1 year is equal to RT + VAC x 1 year except in unfavorable histologies. UH: use VAC
- Groups III-IV: no benefit to adding adriamycin to VAC+RT except in unfavorable histologies.
- Better parameningeal outcomes than with IRS1 with prophylactic WBRT for high risk patients.
- Chemo alone for special pelvic sites with VAC is not adequate. Bladder preservation only 22% because of inadequate response.
Both of these studies demonstrated that the prostate was shown to have the highest risk of lymph node involvement at 40%.
IRS-III This study demonstrated that WBRT prophylaxis did not improve outcome or CNS relapse .
- Group I-II unfavorable histology: Improved with Vincristine, Adriamycin, cyclophosphamide alternating with VAC + RT than VAC or VC + RT (A in VAC is actinomycin D
- Group I favorable histology did not get RT in IRS III. Group III special pelvic sites did not get RT if CR to chemotherapy
- Group II-III favorable site VA+RT adequate
- Group II-III unfavorable site and Group IV (mets) Favorable/unfavorable histology: VAC+RT -- no benefit to adding adriamycin
- Prophylactic cranial irradiation did not reduce CNS relapse.
- there was improved bladder preservation rate and OS in multimodality treatment of special pelvic sites.
- Radiation was not given to Group I favorable histology and Group III special pelvic sites if a CR after chemotherapy.
IRS III Special Pelvic Sites: IRS-III examined special pelvic sites (ie vagina, bladder dome, and uterus) and (bladder neck/trigone and prostate). Treatment and findings were:
- Pelvic I: Bladder dome, vagina, uterus:
- V/Adria/C alternating with VAC for 2 years → 2nd look laparotomy at 20 weeks: IF PR then RT at week 20 + Adriamycin/VP!6 x 2 cycles; IF CR then continue chemotherapy without Radiation
- Pelvic II: Bladder neck and trigone, prostate: V-Adriamycin-C alternating with VAC for 2 years → RT at week 6 → second look surgery at 20 weeks.
- Bladder preservation rates was 60% from 25% (in IRS I-III) and overall survival rate was 83%, up from 72%.
IRS IV focused on improving outcomes for Group III (R2) by adding isfosfamide and etoposide with BID radiation at 1.1 Gy BID to 59.4 Gy.
- QD radiation therapy remains standard at 1.8 Gy/day to 50.4 Gy.
- VAC remains standard. even for alveolar type.
- For Group IV (mets) VAC/IE is now standard.The study compared IE v. melphalan
Prophylactic WBRT was used in IRS I-III to 30 Gy with intrathecal chemotherapy. All started on Day 0. IRS-IV started on day zero but did not treat the whole brain, involved field only in high risk parameningeal sites. IRS-VI (present study) has omitted radiation on day zero in favor of delayed radiation in parameningeal high risk sites with base of skull invasion or CN palsy. Radiation therapy now starts in week 4 except for intracranial extension in intermediate risk and metastatic parameningeal disease.
Wolden Reviewed IRS I-III RT v. No RT in Group I patients (Woldon, 1999, JCO)This analysis showed only a trend to improved Failure Free Survival (FFS) and on overall survival with RT in favorable histology patients. However, in unfavorable histology patients radiation therapy improved FFS and overall survival.
IRS-VI is now underway. Low risk patients on the IRS-VI study are in two subsets:
- Subset I: (treated with VA + RT on IRS III-IV): Stage 1 (favorable sites) Group I-II; orbit Group III, Stage 2 Group I-II -- Now treated with reduced chemotherapy VAC x 4 cycles → VA x 4 cycles + RT
- Favorable histology Group I Stage 1-3 did not get radiation at al -- chemotherapy only.
- Subset II (VAC + RT on IRS III-IV): Stage 1 Group III (non-orbit sites); Stage 3 Group I-II: Now treated with VAC x 4 cycles (reduced chemotherapy) → 12 cycles of VA + RT
the major study questions for IRS - VI for intermediate risk patients are type of chemotherapy: VAC vs. VAC alternating with vincristine/irinotecan and the timing of radiation therapy. Should radiation therapy begin at week 4 or week 10?
For high risk (metastatic disease) the IRS - VI study questions are does VAC alternating with IE in dose dense fashion improve local control and does using vincristine/irinotecan with Radiation Therapy improve local control?
Radiation therapy
Timing
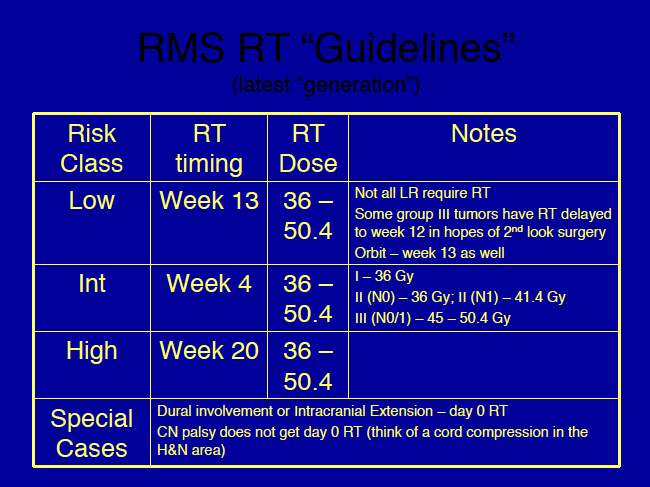
IRS-V Radiation therapy timing in IRS V is different for each risk group:
- Low Risk: RT starts Week 3
- Intermediate Risk: RT starts week 12
- High Risk: RT starts at week 15
- Special Case: Intracranial extension or dural involvement: Day 0
- CN palsy does not get Day 0 RT.
Secondary questions for IRS - VI include radiation dose: Is 36 Gy adequate for N0, R1 and is 45 Gy adequate for orbital RMS? A retrospective review by Mandell in 1990 (JCO) failed to demonstrate a difference between < 40 Gy and > 40 Gy in 32 Group II (R1) patients in local control at MSKCC.
Remember: No RT for favorable histology Group I (R0)!
Nodes
Also remember: Radiation is NEVER omitted in Node Positive disease. Radiation doses in node + patients are 41.4 Gy in R0 (Group I) resections. If gross disease is present or suspect then the dose is carried to 50.4 Gy
All patients with initial nodal involvment, regardless of response to induction therapy or second look surgery must get radiation therapy to 41.4 Gy, if R0/R1 resection (Group I/II). If there is suspected gross disease then treat to 50.4 Gy.
General Techniques and Information -- "Guidelines"
Radiation therapy should be held for ANC< 750, platelets < 75 and for uncontrolled infection or Hb < 10. Radiation may be restarted after these have normalized. If the Hb or platelets are the problem, then chemotherapy should be held or modified until after radiation is completed.
- GTV -- Initial volume at diagnosis
- CTV -- 1.0 cm margin
- Consider CTV-2 for radiographic response to treatment:
- CTV2 -- after 36.0 Gy has been delivered to CTV-1
- CTV2 = GTV + 0.5 cm margin
- Consider CTV-2 for radiographic response to treatment:
- PTV -- 0.5 cm
- This varies by institution
- PTV2 is necessary if CTV2 is used.
Wolden (2005 IJROBP) reviewed IMRT in head and neck patients. A review of 28 patients with 21 parameningeal sites were treated with IMRT with a 1.5 cm margin, and a median dose of 50.4 Gy. Excellent local control at 95% was noted with IMRT despite reduced margins. Wolden noted comparable acute toxicity.
Delayed Treatment
IRS-V provided for dose modifications if treatment were to be delayed. These modifications are:
- < 2 week delay: no change in dose.
- 2- 3 week delay: 1.8 Gy additional dose
- > 3 week delay: 3.6 Gy
Para-aortic node techniques
Treat AP/PA to 36 Gy, then off cord to 50.4 Gy
Extremity Site Techniques
Extremity sites must consider these concerns:
- Evaluate the need to treat regional lymph nodes. (UE: axillary LN, LE: ing/fem LN
- Include scars and drains in the radiation field
- Try to spare a strip of skin and a protion of the joint/epiphysis
"Special" Sites: Vagina, Cervix, Uterus
If a complete response after induction chemotherapy with a Group III N0 tumor in these sites no radiation therapy is required.
For embryonal (favorable risk histology) Group I Stages I-III no radiation therapy is indicated. For unfavorable histologies and Group I 36 Gy is given.
Orbit and parameningeal disease are not recommended for surgery and are treated with radiation and chemotherapy.
Lymph node dissection is indicated for GU ( paratesticular and bladder) sites, in which pelvic and para-aortic lymph node dissections are recommended, and in the extremities and trunk, where the axillary or inguinal/femoral nodes are dissected.
High Risk Disease (mets)
If a patient is high risk the question arises: should the primary be treated with radiation therapy. At Johns Hopkins, the preferred approach is to treat the primary disase and let the patient complete the chemotherapy course. Then metastases are treated with radiation. A recommendation is to consider treating the metastases if there is limited bone marrow in the treated field.
The entire PTV should be covered by the 95% isodose surface and no more than 10% of the PTV should receive > 110% of the prescription dose.
Dose standards for the COG include the following criteria:
- Minor Deviation: PTV:95% IDV covers < 90% of the PTV but between 90% and 100% of the CTV.
- Minor Deviation: PTV receives &110%
- Major Deviation: 95% IDV covers < 90% of the CTV volume.
Dose
St Jude (Regine IJROBP 1995) data suggests taht for 24 CG II patients a local control rate of 89% with < 40 Gy was not statistically different from > 40 Gy (100%). An additional study at MSKCC (Mandell 1990 JCO) noted for 32 patients treated with various doses also found that local control for doses < 40 Gy was equivalent to doses ≥ 40 Gy. This is used as a rationale for including a test dose of 36 Gy in the IRS-VI trial.
Specific Sites: Unfavorable
Bladder and Prostate RMS
Bladder and prostate RMS account for about 50% of all GU RMS. Bladder alone (when it can be identified as the source) indicates a more favorable prognosis. Frequently, bladder and prostate sources of origin cannot be identified. RMS of this site tends to occur in the very young (age < 5 years at diagnosis). Signs include: incontinence, retention, dysuria, polyuria. Regional lymph nodes involvement is common (20+%) at this site, particularly for prostate origin. Hypogastric or external iliac nodes are most commonly involved. Only 15% have distant mets at diagnosis.
Goals of Treatment
The treatment of prostate/bladder RMS is to use multimodality therapy with organ preservation (bladder) to cure. Radical surgeries are no longer used. Neoadjuvant chemotherapy ± radiation therapy is used if surgery cannot achieve a complete (Group I/R0) resection up front with bladder preservation.
IRS-I enrolled 64 patients with bladder/prostate disease and initially were managed with pelvic exent/anterior exenteration. Chemotherapy followed for most patients ± radiation therapy. This treatment led to high rates of local control and survival but led to an effort to reduce the morbidity of surgery. IRS-1 bladder preservation rate was 23% and most recurrences were local with pelvic exenteration used for salvage therapy. Lymph node dissection for node and adjuvant radiation therapy in node positive patients did provide a benefit.
IRS-2 attempted to substitute chemotherapy (more intense dosing) for surgery, but the bladder intact 3 year survival rates were the same as the initial IRS-1, despite a high initial rate of complete response due to higher rates of recurrence.
IRS 3 administered RT routinely to all patients at week 6 after induction chemotherapy initiation, execept in those patients where a complete resection was possible. This was an attempt to eliminate the local failure problem of the first two studies. This resulted in a 4 year bladder preservation rate of 60% and survival of 90% for patients presenting with local or regional disease. Heyn et al showed that 54% ultimately retain their bladders.
Paratesticular Sites
Paratesticular sites are less common GU sites than bladder/prostate GU sites accounting for about 1/3 of all GU RMS. Intrascrotal paratesticular disease arises from the distal area of the spermatic cord and may invade the testes or surrounding tissues. Presentation is usually a painless scrotal mass that does not transilluminate. Biopsy should be performed (inguinal so as not to violate the scrotum). Inguinal orchiectomy is preferred with high ligation of the spermatic cord at the level of the inguinal ring. If a trans-scrotal orchiectomy is performed, the patient is considered to be Group II. Resection of the violated scrotal area is then recommended and radiation or hemiscrotectomy is indicated. Paratesticular RMS has a high rate of spread to the lymph nodes, first to the para-aortic lymph nodes, following the spermatic cord into the renal hilar space. Nearly all are embryonal types (favorable histology).
Radiation doses are 50.4 Gy to the PALN if there is gross residual disease (R2/Group III) or 41.4 Gy if R1/Group II if there is pathologic confirmation of tumor response. All patients with node positive disease get radiation.
Outcomes
Most treatment failures are local. Local failures are greater than distant failures. Lymph node positivity is the most important predictor of local failure.
Per the IRS-IV, favorable prognostic indicators include ≤ 2 metastatic sites and embryonal histology. (Breneman 2003, JCO)
Toxicities of Treatment and Organ Dose Constraints
The dose constraint to the whole kidney is 19.8 Gy.
The dose constraint to the whole liver is 23.4 Gy.
The dose limit to the optic chiasm is 46.8 Gy.
The dose limit to the whole heart is 30.6 Gy.
The dose limit to the abdomen and pelvis is 24 Gy at 1.5 Gy/fraction.
These constraints are described in detail and are derived from the IRS-VI studies.
The major side effect of VAC chemotherapy is veno-occlusive disease of the liver.