Early Stage (I/II) Non-Small Cell Lung Cancer
Background
Demographics and Natural History
In 2009, there were 219,000 newly diagnosed lung cancer cases in the US. These cases accounted for > 160,000 deaths. This disease accounts for more deaths than all colorectal, breast and prostate cancers combined. The 5 year overall survival rate for lung cancers remains dismal at 15%.
Blacks have the highest incidence of lung cancer. Males are also historically at greater risk, but as females entered the smoking pool, this risk is becoming more normalized, with female risk increasing.
The primary cause is tobacco smoke. Three other environmental exposure risk factors are strongly associated with lung cancer:
- Radon gas
- Asbestos. Smoking and asbestos exposures are synergistic or at least multiplicative
- Occupational exposure to arsenic, bis-chlormethyl ether, hexavalent chromium, mustard gas, nickel, polycyclic aromatic hydrocarbons
Heavy smokers have enormously increased relative risk of developing lung cancer. The relative risk is at least 20 RR, based on an ACS cohort study. Quitting smoking helps lower that risk to less than 9 fold, or around half that of those who continue to smoke. Passive smoking is also implicated in that the risk to passive smokers from heavy smoke is 1.2 - 1.3 times that of those who are not exposed to smoke on a regular basis. Less than 20% of smokers actually develop lung cancer based on data from a trial: Carotene and Retinol Efficacy Trial. The 10 year cancer risk was 1% - 15%. Adenocarcinoma is the cancer subtype least associated with tobacco use.
Screening
Screening for lung cancer in high risk populations has been studied and remains controversial. Early screening studies used chest x-rays, but ultimately although earlier diagnosis was made it appeared to be a result of earlier diagnosis with no improvement in outcomes, with longer lead times to outcomes. CT based screening is being studied by the IELCAP which reported in 2006 that out of 27,456 patients screened with CT, 74 were found to have cancer (detection rate of 0.3%) and 86% were stage I. OS-10 was 93% in Stage I patients who underwent resection at diagnosis. OS-10 was 82% for all patients diagnosed by CT.
Presentation
The most common presenting signs of NSCLC are Dyspnea, cough and weight loss. Other signs include chest pain, including pleuritic or chest wall pain or angina like pain, and hemoptysis.
The most common presenting stage is metastatic disease found in about 1/3 of all patients. Lung cancer most commonly metastasizes to the bone, adrenals and brain.
Lung cancers also present with paraneoplastic syndromes. Hypercalcemia of malignancy due to PTHrP, SIADH leading to hyponatremia, Cushings, Lambert-Eaton Syndrome (decreased Acetylcholine release causing muscular weakness), and other neurologic disorders.
Lambert-Eaton syndrome is caused by circulating antibodies against the pre-synaptic P/Q calcium channel. Lambert Eaton strength improves with serial effort due to increasing acetylcholine in the neuromuscular junction. In myasthenia gravis, the muscle weakens with repeated effort.
The site and some characteristics of disease are dependent on the histologic subtypes.
- Squamous cell carcinomas tend to be central.
- Adenocarcinomas tend to be peripheral.
There are three distinct adenocarcinoma variants of the lung:
- bronchoalveolar
- acinar
- papillary
Giant cell and clear cell lung cancers are associated with large cell variants.
TTF-1 staining is associated with these histologic subtypes:
- Adenocarcinomas
- Non-mucinous bronchoalveolar carcinomas,
- Neuroendocrine tumors (such as SCLC, carcinoid).
TTF-1 positivity is rare in squamous cell carcinomas and if found, a thyroid cancer must be excluded.
Genetic Markers
The most clinically significant genetic marker to date is EGFR mutation on chromosome 19. This mutation results in a constitutive active receptor. EGFR receptor activation is seen in only about 10% of all lung cancer patients who appear to share common attributes:
- Never smokers (30% - 70%)
- Adenocarcinomas
- Asian women
These markers predict for a higher response rate to tyrosine kinase inhibitors of about 80%. TKIs are gefitinib (Iressa), and erlotinib (Tarceva). If the EGFR mutation is T790M this point mutation conveys TKI resistance. EGFR amplification on FISH is a good predictor of response to gefitinib/erlotinib TKIs.
KRAS mutations predict for resistance to platinum based chemotherapy.
Workup and Staging
the initial workup for lung cancer includes:
- a focused H&P including weight loss > 5% over the prior 3 months
- KPS
- Tobacco use history
- Neck exam for N3 disease
- Labs: CBC, CMP
- Imaging: CT Chest including adrenals or PET/CT; MRI for paraspinal/superior sulcus tumrors; MRI Brain for presumed stages II/III
- Endoscopic biopsy or FNA biopsy
- mediastinoscopy or endobronchial ultrasound for suspected hilar/N2 nodes
- PFTs prior to treatment
Sputum cytology has been used but has a low sensitivity at < 70%. It is highly specific and requires a minimum of 3 sputum samples. Accuracy increases with increasing number of specimens.
PET/CT has better sensitivity at 83% over CT at 64% and better specificity at 91% compared with CT at 74%.
- PET/CT: Sensitivity: 83% Specificity: 91%
- CT: Sensitivity 64%, Specificity: 74%
About 10% - 20% of patients will have false positive N2 nodes on PET/CT. The positive predictive value (TP/(TP+FP)) is around 80%. N2 positive nodes by PET/CT need pathologic confirmation prior to ruling out definitive surgery. The incidence of false negative mediastinal (N2) nodes based on PET/CT is 5% - 16%. The negative predictive value (TN/(TN+FN)) is 95%. Clinical T1 lesions do not need mediasinoscopy evaluation, although this is debated. PET/CT detects occult lung cancer mets in around 6% - 18% of cases.
If a PET/CT is ordered, there is no need for a bone scan. PET is just as sensitive as a bone scah but more specfic. The PET scan may give a false positive, particularly where there was a pre-existing bone injury, sometimes dating back years, so pathologic confirmation may be necessary in some circumstances, especially a solitary positive.
The most important clinical characteristic of a solitary pulmonary nodule is the nodule size and its change history..If the nodule is unchanged and stable over time, it is more likely to be benign. Other factors include smoking history and the appearance of the nodule on CT, particularly spiculated margins.
Adenocarcinoma has a worse prognosis than squamous cell carcinoma based on equal staging. Adenocarcinomas have greater tendancy to metastasize, particularly to the brain. Large cell carcinomas have a similar natural history to adenocarcinomas.
Staging AJCC 2009
| Stage | Primary Tumor |
|---|---|
| T1a | tumor ≤ 2 cm |
| T1b | tumor 2 - ≤ 3 cm |
| T2a | Tumor > 3 - ≤ 5 cm |
| T2b | tumor > 5 cm - ≤ 7 cm |
| T3 |
|
| T4 | Any size that invades any of the following:
|
| N | Node Status |
|---|---|
| N1 | Ipsilateral hilar or peribronchial nodes |
| N2 | ipsilateral mediastinal nodes or subcarinal nodes |
| N3 | contralateral mediastinal or hilar nodes or supraclavicular/scaline nodes. |
| M | Status |
|---|---|
| M1a | Separate tumor in contralateral lobe or malignant pleural/pericardial effusion |
| M1b | Distant Metastases |
AJCC 2009 Stage Grouping
| T | N0 | N1 | N2 | N3 | M1 |
| T1 | IA | IIA | IIIA | IIIB | IV |
| T2a | IB | IIA | IIIA | IIIB | IV |
| T2b | IIA | IIB | IIIA | IIIB | IV |
| T3 | IIB | IIIA | IIIA | IIIB | IV |
| T4 | IIIA | IIIA | IIIB | IIIB | IV |
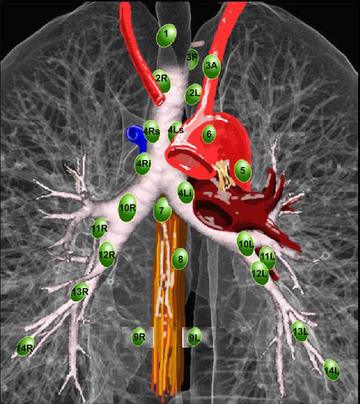
Nodal Assessment
Prior to treatment nodes should be assessed. Nodal stations are based on the Mountain Nodal labelling which has been supplanted by the ILSG
 |
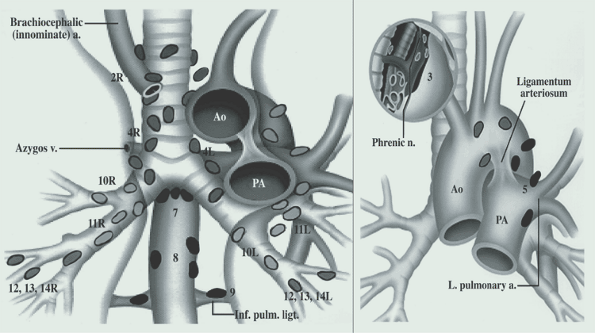 |
Nodal assessment technique depends on the location of the lymph nodes in question. Stations 5 and 6 are aortico-pulmonary window nodes and aortic nodes. They are not accessible via mediastinoscopy and require an anterior mediastinotomy or Chamberlain procedure. The two nodal procedures used are mediastinoscopy or endobronchial ultrasound for non-level 5/6 nodes and the VATS (video assisted thoroscopy) or anterior mediastinotomy .
- Mediastinoscopy
- evaluate R/L Level 2, 4, 7 nodes
- Endobronchial U/S
- evaluate R/L level 2, 3, 4, 7 and 10 nodes
- VATS or anterior mediastinotomy (Chamberlain Procedure)
- Evaluate Level 5 and 6 nodes.
Mediastinal nodes should be assessed when:
- PET/CT or CT nodes are positive.
- All Superior sulcus tumors
- If T3 or central T1-T2 lesions are seen
Pulmonary Function Tests
Pulmonary function tests are critical tests to assess the ability to undergo treatment in lung cancer. Many patients have decreased pulmonary functions as a direct result of smoking leading to their cancer. Many have marginal respiratory function at presentation.
Pulmonary function tests that indicate the need for further testing include:
- FEV1 < 80% of predicted for age and size
- DLCO < 80% of predicted.
Patients not meeting these criteria (which are nearly all lung cancer patients) should undergo additional lung functional testing, including:
- Quantitative V/Q scans if available. Note SPECT may be an alternative.
- Exercise testing
The absolute minimum FEV1 for pneumonectomy is > 2 L. The absolute minimum for lobectomy is > 1.5 L. Any patient with FEV1 < 1.5 L may be a candidate for a wedge resection. The marginal FEV1 should be ≥ 40% of the predicted value.
Candidates that are high risk for surgical morbidity are:
- pCO2 < 45 mm Hg
- pO2 < 40 mm HG
- Preop FEV1 < 40% of predicted value
- Poor exercise tolerance
- DLCO < 50% of predicted
- Post Op FEV1 < 0.71 L or < 30% of predicted
- Cardiac problems (LVEF < 40%, AMI in less than 6 months, arrhythmias)
- Obesity
Other factors which predict for postoperative complications, including mortality, infections) include active smoking.The risk is 6 times higher. Poor nutrition, advanced age and poor pulmonary function.
All patients should quit smoking at least 6 weeks prior to surgery and be evaluated for nutrition.
About 5% - 25% of Stage I (T1 - T2a (tumors ≤ 5 cm) N0) patients are clinically upstaged at surgery.
Other poor prognostic factors include:
- KPS < 80
- Weight loss > 5% in 3 months
- Age > 60
Treatment and Prognosis
The general treatment paradigm for Stage I/II NSCLC is to operate in medically operaable patients. Surgical resection (lobectomy) + mediastinal LND → adjuvant chemotherapy for Stage II disease.
For medically inoperable patients the general treatment paradigm for Stage I/II disease is if N0, consider definitive hypofractionated stereotactic body radiation therapy. Otherwise use definitive conventional radiation therapy alone.
Surgical Management
Surgical options to resect a T1/T2 tumor (size ≤ 7 cm) include:
- Wedge or segmental resection
- Lobectomy
- Pneumonectomy
Wedge resections are not considered a cancer operation, however they enjoy a reasonable success rate of 82% local control. Lobectomy provides better local control at 94%, based on a randomized controlled trial from the Lung Cancer Study Group,LCSG 921. Lobectomy is preferred when feasible.
For at T1N0 NSCLC the estimated local control for wedge/segmental resection ± intraoperative brachytherapy is 97% with brachytherapy and 83% without brachytherapy. The outcomes may be similar to lobectomy. (J.Thorac.Onc. 2005, Fernando)
The same factors in the development of lung cancer continue to operate in lung cancer patients post resection. Up to 30% will develop a second primary disease.
The OS-5 for T1N0 (Stage IA) is 80%. The OS-5 for T2N0 (Stage IB/IIA) is 68%.
The natural history of untreated lung cancer is fast and relentless. For those who refuse treatment, the OS-5 is 6%, the Cause Specific Survival is 22% and the Median survival without treatment is 13 months.
Adjuvant Chemotherapy
The indications for adjuvant chemotherapy after definitive (complete) resection for Stage I/II NSCLC include:
- Stage II-IIIA disease (Nodes and smaller tumor or large tumors/CW invasion, etc)
- N1 Disease (NCCN Category I recommendation)
- T2N0, especially if tumor > 4 cm per the unplanned CALGB 9633 analysis.
Chemotherapy adds a 5% OS-5 benefit based on the LACE meta-analysis (Pignon 2008 JCO)
MRC-UK LU22/EORTC 08012 examined pre-op chemotherapy compared to surgery alone (2007, LANCET) and found no survival benefit. EORTC did find a 31% downstaging rate. A meta-analysis showed that the 5% survival gain for preop chemotherapy was the same as post-op chemotherapy. There does not appear to be a role for pre-op chemotherapy based on these data.
There may be a role for full mediastinal dissection over selective nodal sampling in early stage lung cancer undergoing resection. A pooled analysis of 3 trials demonstrated a 4 year overall survival benefit in Stage I - IIIA NSCLC. In this analysis, mediastinal dissection includes 2R, 4R, 7 (subcarinal) 8R/L, 9R/L, and 5, 6.
Radiation Therapy
Radiation therapy is indicated in early stage lung cancer (Stage I/II) in the post operative setting when there are postive surgical margins, extracapsular extension or unexpected N2 disease. The NCCN recommendations in these settings recommend concurrent chemotherapy-radiotherapy for positive margins and Sequential chemo-radiation therapy for N2 disease (chemo → radiation). Chemotherapy → RT will also be recommended for ECE.
A randomized Italian study (Trodella Radiother. Onc. 2002) compared post operative radiation therapy to observation. This study examined 104 patients with Stage I disease randomized to PORT or observation. PORT encompassed the bronchial stump and the ipsilateral hilum, (mean area 50 cm2 or 7 x 7 field). Radiation to 50.4 Gy was delivered. Local failure rates improved from 23% to 2% with PORT.There was also a trend toward improved survival at 67% compared to 58% in observation alone arm. There was minimal toxicity and pulmonary function remained stable.
Maximum radiation doses delivered are generally accepted to be 84 Gy for Stage I/II NSCLC, if lung dose volume (V20, MLD, V13) are respected. Spring Kong reported on a dose escalation study which indicates that the tumor dose may be less critical than the lung dose constraints with highly conformal fields.
RTOG 9311, which included Stage III patients as well as early patients reported the 2 year local recurrence rate for radiation aolne using standard fractions was 50% - 70%
The elective nodal failure rate (in RTOG 9311) was < 10%. In most series, of Stage I lung cancer treated with SBRT, the regional nodal failure rates ranged from 5% - 10%. An Indiana University dose escalation study (Hoopes 2007 Lung Cancer) in Stage I disease demonstrated elective nodes as site of first failure of under 10%. Therefore, there is no role for elective nodal radiation in Stage I/II NSCLC treated with definitive radiation therapy alone.
Stereotactic Body Radiation Therapy
SBRT has been shown, using hypofractionation for Stage I NSCLC to improve local control at 3 and 5 years. LC-3 ranged from 85% - 95% and OS-3 was 55% - 91%.
A Biologic effective dose of > 100 Gy gives a local control of 92% and an OS-5 of 72% in Stage I lung cancer treated with SBRT. If the BED is < 100 Gy local control drops to 57% and OS-5 drops to 50%. (Onishi 2007 JTO).
Distant metastases is similar to surgical resection rates at 15% - 20%.
RTOG 0236 used SBRT at 20 Gy x 3 fractions (to 60 Gy) (Timmerman 2010 JAMA)without homogeneity corrections. Dose was given over 1.5 - 2 weeks and when inhomogeneity was considered, 18 Gy x 3 fractions was administered. Findings:
- OS-3 55.8%
- DFS-3 48.3%
- LC-3 90.6%
Lesions within 2 cm of the proximal bronchial tree are not good candidates for hypofractionated radiation at 20 Gy x 3 fractions per the RTOG 0236 findings, due to the risk of Grade 3 - 5 toxicity. Chang (MDACC) proposed a reduced dose of 12.5 Gy x 4 fractions to 50 Gy, At 17 months, LC was 100% with Grade 2-3 dermatitis and chest wall pain in 11%. There was no pneumonitis in newly diagnosed Stage I patients. Johns Hopkins use 4 Gy x 15 fractions to 60 Gy (BED3 140 Gy) or 5 Gy x 12 fractions to 60 Gy (BED = 160 Gy).
There are several other studies presently addressing the role of SBRT in the treatment of resectable early stage lung cancer. They are:
RTOG 0618 Phase II operable Stage I/II NSCLC presently accruing using 18 Gy x 3 fractions.
ROSEL (Dutch) Phase III SBRT v. surgery for operable Stage I NSCLC. Radiation dose is 20 Gy x 3 for T1 lesions (≤ 2 cm), 12 Gy x 5 for T2 (≤ 7 cm) and 7.5 Gy x 8 fractions for central tumors. the primary endpoint is LC-2 and LC-5, quality of life and cost.
STARS (Accuray/MDACC) Phase III SBRT (cyberknife) v. surgery in operable stage I patients. Radiation therapy is 12.5 Gy x 4 (60 Gy, BED10= 135 Gy, BED3=310 Gy) for central lesions and 16.7 Gy x 3 for peripheral tumors. Primary endpoints are OS, DFS and toxicity at 3 years.
Dose and Volumes
Post operative radiation therapy for microscopically positive (R1) margins is to 54 - 60 Gy. For macroscopic (R2) margin, use 60 - 70 Gy.
Toxicity and Dose Constraints
Considerable research has been done into the dose constraints of lung cancer treatment. Spring Kong and Andrew Turrisi (UMich) have done significant work in this area.
Lung cancer fullow up includes an H&P + CT chest with contrast every 4 - 6 months following completion of treatmetn for years 1 and 2. Then noncontrast CT annually for years 3 - 5. Smoking cessation should be continued.
PET/CT may not normalize due to inflammatory response after SBRT. A normal SUV is not required to demonstrate good clinical response. There are a number of prospective and retrospective studies which demonstrate there is no evidence of a correlation of sustained elevated SUV and recurrence in this setting. (Timmerman, 2009 IJROBP, Henderson IJROBP 2009))
15% of those treated with SBRT for early stage lung cancer have grade 3 - 5 toxicities in a review of 15 studies by Sampson in 2006 (Sampson 2006 Sem. Rad. Onc). Timmerman ( RTOG 0236 2010 JAMA) reports 16.3% experienced Grade 3 - 4 toxicity but no grade 5 toxicity.
A Univ. Indiana Phase II SBRT study (Timmerman 2006 JCO) reported Grade 3 - 5 toxicities after SRBT for early lung cancer. They found location and tumor size were important with 46% toxicity in central/hilar regions and 17% in peripheral regions. Tumor size with GTV > 10 cc had 8 x the risk of grade 3 - 5 toxicity.
DLCO and FEV1 were minimally changed with SBRT (Stephans 2009 JTO) There was no association seen between central and peripheral locations or doses delivered.
The major toxicities of SBRT treatment include:
- pneumonitis
- lung fibrosis and consolidation
- cough
- dermatitis
- Chest wall pain
- Esophagitis
Pneumonitis:
- Grade 1: Imaging changes, asymptomatic
- Grade 2: symptomatic with need for steroids
- Grade 3: dyspnea at rest with the need for supplemental O2
- Grade 4: hospitalized and intubated
- Grade 5: Death
Dose Constraints
The V20 (volume of lung receiving ≥ 20 Gy - CTV) constraint is < 37%
The Mean Lung Dose (MLD should be < 20 Gy
Heart Constraints: V45 < 67%. V66 ≤ 33%
Esophagus: The entire esophagus should be included in the dose calculation from the thoracic inlet to the GEJ. Kong et al looked at dose/volume/toxicity and found that esophageal tolerance may be quite high. A non-related dose escalation study in esophageal cancer was stopped prematurely due to excess mortality in the dose escalation arm, but it is noted that most of the deaths occured well below even standard doses were delivered to the esophagus. Kong reported (IJROBP) that initial esophageal constraints were 65 Gy, then increased to 72 Gy then 80 Gy due to lack of toxicity.
Presently, esophageal recommendations are:
- Mean dose < 34 Gy
- V60 < 33% (try to minimize as much as possible)
- V55 < 66%
- V45 ≤ 100% (ie the whole esophagus dose should be kept under 45 Gy)
Heart Constraints:
- V60 < 33%
- V45 < 67%
- V40 < 100% (less than 40 Gy to the whole heart).
Brachial Plexus constraint is < 66 Gy with conventional fractionation.
For SBRT, the maximum BED per Timmerman (206 JCO) is 180 - 210 Gy for centrally located tumors.
Keeping the BED ≥ 100 Gy may be sufficient for local control and may avert toxicities for central lesions. Note: Timmerman and Senan used BED10 (α / β = 10 for tumor response). The linear quadratic equation may not be entirely applicable to extreme doses per fraction.