Prostate Cancer
Prostate cancer is divided into low, intermediate and high risk groups by most experts. There are about 230,000 cases and 27,000 deaths from prostate cancer annually in the United States. Approximately one in six men will be diagnosed with prostate cancer in their lifetimes. Prostate cancer is the second most common cause of cancer death behind lung cancer and is essentially tied with colorectal cancer.
Between 12 and 28% of men with newly diagnosed prostate cancer will have clinical stage T3 disease and higher. Clinical Stage T3 disease includes extra-capsular extension and/or seminal vescicle involvement. Nearly 1/3 will have Gleason Scores ≥ 7. In the US, 1/10 of all newly diagnosed prostate cancers is Gleason ≥ 8.
In men with PSA (pre-biopsy) ≤ 4 ng/ml, the risk of Gleason Score ≥ 7 is PSA x 2.
Black males appear to be at highest risk for prostate cancer and tend to present with more aggressive disease than other groups. They tend to have higher Gleason Scores, and a more advanced stage at diagnosis. Asian men are at lowest risk with a 30 - 50 fold difference in incidence observed between blacks and asian groups.
There is no identified causal relationship between vasectomy and development of prostate cancer. The NIH concluded that there is no convincing data supporting a causative relationship between vasectomy and interval development of prostate cancers. A causitive relationship has not been established.
An autopsy study of men who died of unrelated causes at Wayne State University examined prostates for evidence of prostate cancer in this population. The found that the incidence of prostate cancer increases with age with Gleason Scores 6 - 7.
| Age | PIN | Cancer |
|---|---|---|
| < 30 | 0.6% | 0.6& |
| 40 - 49 | 19.2% | 0% |
| 50 - 59 | 40.3% | 23.4% |
| 60 - 69 | 61.2% | 34.7% |
| 70 - 81 | 45.5% | 45.5% |
Clinical factors associated with the diagnosis of prostate cancers include:
- Advanced Age
- African American ancestry
- Previous history of biopsy confirmed PIN (prostatic intra-epithelial neoplasia), and especially high grade PIN
- Obesity
- High intake of dietary fats
Prostate Anatomy and Natural History
There are four zones of the prostate:
- Peripheral zone (2/3 of all prostate cancers arise in this zone)
- Central Zone
- Transition Zone (Benign prostatic hyperplasia develops in the TZ)
- Anterior fibromuscular stroma
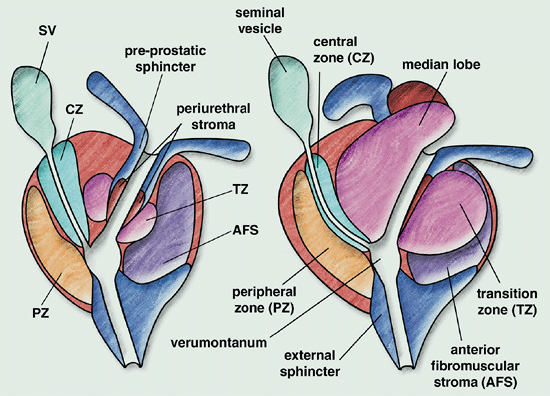
The apex of the prostate is not clearly identifiable. Some argue that prostate does not have a true capsule but an outer fibromuscular band that transitions continouosly to periprostatic tissues and organs. The transition at the apex is particularly difficult to identify.
In prostate cancers with extracapsular extension, this is most commonly seen at the neurovascular bundles in the posterio/lateral aspect of the prostate.
Median lobe hypertrophy refers to a characteristic transitional zone hypertrophy that mushrooms superiorly into the rest of the prostate and bladder. This hypertrophy originates in the TZ and compresses the central zone, reducing its size in older men. At the posterior aspect of the prostate the neurovascular bundle runs in the lateral aspects. This bundle is responsible for erectile function of the penis.
The normal prostate histology consists of secretory PSA producing cells which involute with androgen deprovation, basal cells which support secretory stem cells, and neuroendocrine cells.
Clinical grading of prostate cancer is done with the Gleason grade and score. The Gleason Grade is a grade assigned to prostate cancer specimens that reflects the microscopic degree of aggressiveness based on the degree of resemblance to normal prostatic tissue. The Gleason Grades are:
- Grade 1: small well-formed glands, closely packed
- Grade 2: well-formed glands but more tissue between them
- Grade 3: darker cells, some of which are invading the surrounding tissue
- Grade 4: few recognizable glands with many cells invading the surrounding tissue
- Grade 5: no recognizable glands; sheets of cells throughout the surrounding tissue.
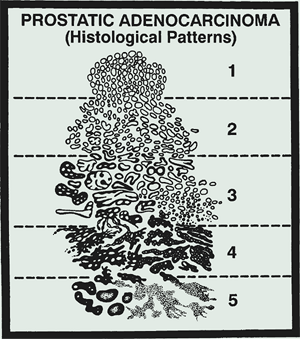
The Gleason score is the sum of the two most prevalent patterns and ranges 2 to 10. Approximately 1/3 of call prostate cancers are higher grade than the biopsy grade in post-prostatectomy specimens. Upgrading the Gleason Score. occurs in 1/3 of the prostates
Black males are at highest risk for development of prostate cancer and they tend to have higher risk and more advanced stage than other groups. Asian men have the lowest risk for the development of prostate cancer. There is a 30 - 50 fold difference between the incidence observied between native Asians and black males.
Clinical Risk and Prognosis
PSA ranges are associated with prostate cancer risk.
- PSA < 4: 5% - 25%
- PSA 4 - 10: 15 - 25%
- PSA > 10: 40 - 67%
PSA values can be affected by manipulation of the prostate, either via biopsy or DRE, infections, cancer of the prostate, benign prostatic hyperplasia, ejaculation shortly before testing and prostatitis. Some cancers do not make much PSA and therefore, the prostate cancer screening includes both a PSA and DRE. PSA features as a determinant of cancer risk include :
- Age (PSA goes up with age)
- PSA velocity
- PSA density
- ratio of free to total PSA
The PSAV (PSA velocity) is a measure of the rate of change of total PSA annually. A psa velocity ≥ 2 ng/mL/year is associated with a higher risk of finding a Gleason ≥ 7 prostate cancer on prostatectomy. The PSA density is the total serum PSA / volume of the prostate gland. An approximation of the elipsoid volume can be determined by measurement of the width x height x length x 0.52. A PSA density > 0.15 ng/ml/cm3 identifies a higher probability of finding prostate cancer on biopsy.
There are two PSA levels an inactive "free PSA" which is the end product and an earlier product. The prostate cancer cells disrupt the basement memebrane and allow precursor forms of PSA to leak into circulation. This decreases the proportion of free PSA. The proportion of free PSA will be lower in men with prostate cancer. A ratio of ≤ 7% free PSA is highly suspicious for prostate cancer. A ratio of free PSA to PSA of > 25% is rarely associated with malignancy.
Screening and Prevention
Until recently, screening was not controversial. The US Preventive Services Task Force, on the eve of the adoption of Obama-care, concluded that there is inusfficient evidece in men under age 75 years to assess the balance between benefits and risk of screening. They also recommended against screening men > 75 years old. This is the same panel that concluded mammography was not useful in women 40 - 50 and could be done every other year. Contrary to this recommendation, the American Cancer Society recommends annual DRE and PSA testing beginning at age 50 with a normal risk of prostate cancer.
A randomized European study comparing PSA screening to no screening found in 182,000 men enrolled and a median follow up of 8 years, the cummulative incidence of prostate cancer was 8.2% in the screened group and 4.8% in the unscreened group. The ratio of death from prostate cancer in the screened group was 0.8 as compared with the unscreened group. (p=0.04). 1410 men need to be screened and 48 prostate cancers need to be treated to prevent one prostate cancer death.
The US Prostate, Lung Colorectal and Ovarian Cancer screening trial, a phase III trial (PLCO) screened 76,700 men with annual PSA and DRE. After 7 years follow up, the incidence of prostate cancer was higher in the screened group (116 cases /10,000 person-years v. 85 cases/10,000 person-years). The incidence of death was similar between the two groups at 2 cases v. 1.7 cases/10,000 person-years
Criticisms of the PLCO trial include:
- 40% - 52% of the control group were screened with PSA testing
- 41-46% of the control group were screened with DRE
- The study median follow up was only 7 years. The European study did not show curve divergence until 8 years.
In the PSA era, there are usually no clinical signs of prostate cancer. At present the most common presentation is an abnormal PSA level without associated symptoms. Bone metastases is the most common site of metastatic spread. Prostate cancer is almost never found in the brain.
Prevention
Finasteride has been studied in a Phase III trial comparing finasteride with placebo given for seven years to test the role of finasteride in the prevention of prostate cancer age ≥ 55 years without evidence of prostate cancer at enrollment. This study demonsrated a incidence of prostate cancer by 25% (from 30.6% to 18.6%). But, it also showed increased risk of more aggressive prostate cancers (Gleason 7-10 accounted for 37% of tumors on finasteride arm compared with 20% on placebo arm). Subsequent follow up studies suggests that finasteride shrinks the prostate, making it easier to detect disease on biopsy, rather than affecting grade.
Staging and Work up
The work up and staging of prostate cancer is focused on genito-urinary signs and symptoms. GU/lower GI symptoms may be clinical presentations of cancer itself and may guide the selection of treatment. The International prostate symptom score is used as a formalized guide to urinary symptoms. This scoring system consists of 7 questions:
- How often in the last month have you had a sensation of not emptying your bladder completely? (0 -5: none - almost always)
- Frequency: had to urinate ≤ 2 hours. (0 - 5)
- Stream interruptions: (0 - 5)
- Urgency (0 - 5)
- Weak Stream (0 - 5)
- Need to push or strain to urinate (0 - 5)
- Nocturia (0 - 5)
Comorbid conditions may inform and alter therapeutic choices. A history of inflammatory bowe disease, hernia repair or previous bowel surgery. Is the patient a surgical candidate or candidate for hormonal therapy? Is the patient presently on α-blockers? New onset boney pain should trigger a bone scan and comprehensive evaluation for bone metastases.
Laboratory studies include PSA, CBC, basic metabolic profile. Consider free PSA if diagnosis is uncertain. Consider alkaline phosphatase to assess for bone mets, if suspicious, clinically.
Prostate biopsy should be performed with trans-rectal ultrasound guidance with a 5 to 7.5 MHz ultrasound transducer in the rectum. A sextant Biopsy directed at the peripheral zone should result in 12 cores of tissue for biopsy. Prostate cancer is usually hypoechoic on trans-rectal ultrasound.
No special imaging studies are repcmmended in the evaluation of a newly diagnosed patient with low risk prostate cancer, unless there are signs and symptoms of boney metastases.
Staging
| T1a | Incidental finding involving < 5% |
| T1b | incidental finding involving > 5% |
| T1c | biopsy due to elevated PSA |
| T2a | tumor involves ≤ 1/2 of one lobe |
| T2b | Tumor involves > 1/2 of one lobe |
| T2c | tumor is bilateral |
| T3a | Extracapsular extension |
| T3b | Seminal vesicle involvement |
| T4 | adjacent organ involvement (bladder neck, external sphincter, rectum, pelvic wall or levator muscles |
| N0 | no nodal metastases |
| N1 | regional nodal metastases |
| M1a | non-regional lymph nodes |
| M1b | bone metastases |
| M1c | other sites |
| pT2a | tumor involves ≤ 1/2 of one side |
| pT2b | tumor involves > 1/2 of one side |
| pT2c | Tumor involves both sides |
| pT3a | extracapsular extension or microscopic invasion of the bladder neck |
| pT3b | seminal vesicle involvement |
| pT4 | involvement of the rectum, levator ani muscles, and/or pelvic wall |
The present (2010) AJCC staging system also considers Gleason score and PSA values in assigning an stage group. The AJCC also has two distinct stage groupings based on whether the disease is clinically staged or pathologically staged, making this revision much more cumbersome in routine use. We have for many years considered PSA and Gleason score in the evaluation of prostate cancer with divisions into low, intermediate and high risk groups, based on the pre-treatment PSA and Gleason Score, however the most recent AJCC stage grouping incorporates these data at the expense of ease of use.
Generally, clinically, prostate cancers categorized as low risk disease (T1-2a, node negative with PSA < 10 and Gleason score ≤ 6) are Stage I. Intermediate risk disease (T1 - T2b with PSA 10 - 20 and Gleason ≤ 7 are Stage IIA. Gleason ≥ 8 or PSA ≥ 20 or involvement of both lobes is Stage IIB, and any ECE/SV involvement is Stage III. T4 or Nodal disease or distant mets are Stage IV.
The most important risk factors for stratification of locally confined prostate cancer are:
- Pre-treatment PSA (< 10, 10 - 20, > 20)
- DRE-defined clinical T-stage
- Gleason Score
The most commonly used prostate cancer prognostic risk groups are defined by Anthony d'Amico's criteria. They are:
- Low Risk
- Stage T1c - T2a, PSA ≤ 10 ng/ml, Gleason Score ≤ 6: 10 year Biochemical Failure Free survival: 83%
- Intermediate Risk:
- T2b, PSA 10 - 20 ng/ml, Gleason Score 7 B-FFS @ 10 years: 46%
- High Risk:
- T2c, PSA > 20 ng/ml Gleason Score ≥ 8: B-FFS@10 years 29%
The Roache equations were derived from prostate cancer patients who had surgical management between 1982 and 1996 in the pre-PSA era. These tools overestimate the risk in the post-PSA era.
Risk of Extraprostatic Involvment: The Roach Equations
- Extra-capsular Extension
- ECE % = (3/2)PSA + 10(Gleason Score - 3)
- Seminal Vesicle Involvement
- SV % = PSA + 10(Gleason Score - 6)
- Lymph node risk
- LN % = (2/3)PSA + 10(Gleason Score - 6)
Clinical imaging using MRI endorectal coils have widely varying estimates of extraprostatic extension reliability between 13% and 95% sensitivity and 49% - 99% sspecificity. Radiologist experience is an important determinant in the quality of the study. For determining the presence of seminal vesicle invasion, endorectal coil MRI sensitivity likewise varies widely from 23% to 80%. The estmiates for specificity of SV invasion are better and vary between 81% and 99%. As a result, there is no consensus on the role of endorectal coil MRI imaging as part of the work up for prostate cancer. If MRIs are ordered as part of the work up a wait of 6 - 8 weeks post biopsy should be considered to avoid post biopsy hemorrhage artifact.
Low-Risk Prostate Cancer
There are a number of alternative management approaches to low risk prostate cancer. They range from watchful waiting to active surveillance to full treatment. There are differences between active surveillance and watchful waiting. Active surveillance in deferral of immediate treatment with definitive treatment given if disease progression is determined. The goal of care is to cure those with progressive disease while avoiding unneeded treatment in patients with clinically non-significant disease.
Watchful waiting forgoes definitive treatment at diagnosis with the goal of care ot provide palliative treatment as symptoms develop. Watchful waiting is reserved for the elderly or those with substantial comorbidities with limited life expectancy.
The Swedish Men's prostate cancer study SPCG-4 was a surgical study in the pre-PSA era that randomized 695 men with T1/T2 prostate cancers to radical prostatectomy or watchful waiting. They found that Surgery improved the 12 year cause specific survival to 17.9% as compared with 12.5% and the distant metastases free survival from 19.3% to 26%. Caution in applying this study is warranted. The study included patients with Gleason 7 - 10.
The underlying premise of active surveillance is that many men have low risk prostate cancer and possibly would not have any adverse clinical consequences from not treating their disease. Active surveillance attempts to delay or ovaid treatmetn for the majority of these men while reserving potentially curative treatment for those who would be likely to exhibit aggressive features. In following active surveillance protocols, Johns Hopkins recommends biannual DRE and PSA screening with annual prostate biopsy. Patients are referred for definitive management for increasing Gleason score and increasing volume of disease as defined by the number of positive cores and the length of the positive cores or percent involved, or upon patient preference.
Patients that might benefit most from active surveillance or watchful waiting are those with limited life expectancies, or those with assymptomatic metastatic disease. There is no consensus on wht group of men, if any, are suited for expectant management in early stage prostate cancer, but in general the following criteria are useful:
- Older age combined with comorbid disease and limited life expectancy.
- Clinical T1 disease
- PSA density ≤ 0.1 ng/ml/cm3
- Gleason score ≤ 6
- ≤ 50% involvement of ≤ 2 of 12 cores on biopsy.
At a median follow up of 3 years Johns Hopkins reported 25% of a cohort undergoing active surveillance undersent definitive management based on progression on biopsy, or patient preference. Of those who elected surgical managment, at the time of follow up, a similar incidence of curabile disease to those who would have otherwise qualified for active surveillance but chose immediate surgery. In a prospective signle arm study of 450 men undergoing active surveillance, the 10 year prostate cancer survival was 87.2% Overall, 30% of the men were found to have progressive disease and were offered definitive therapy.
The treatment of low risk prostate cancer in otherwise healthy men with no adverse GI/GU symptoms include:
- Active surveillance in carefully selected patients
- Radical prostatectomy
- Radiation therapy: either External beam therapy or brachytherapy permanent implant.
There have been, to date, no randomized controlled trials to compare surgery to external beam radiation therapy to brachytherapy in the treatment of prostate cancer. There have been a number of trials that have failed to accrue for low risk prostate cancer. At present one trial of 200,000 men in the UK the ProtecT trial has recently closed to accrual, with over 2500 men in the study with localized prostate cancer. 500 men have been randomized toU one of three treatments within the study:
- Active surveillance
- Surgery
- Radiation
In the absence of randomized controlled studies, retrospective studies have been performed. These numerous retrospective reviews have suggested similar outcomes with surgery, external beam radiation therapy and brachytherapy in low and low-intermediate risk prostate cancer. D'Amico reviewed low risk prostate cancer patients and found no difference in 5 year biochemical failure free survival at 88% in men treated with prostatectomy, LDR brachytherapy and external beam radiation therapy with doses > 72 Gy.
Pat Kupelian at Cleveland Clinic and Memorial Sloan Kettering did a similar study and found similar results: biochemical failure free survival was similar to d'Amico at 81% - 83% for men treated with prostatectomy, low dose brachytherapy, or external beam radiation therapy to doses > 72 Gy. Further Kupelian found that for doses < 72 Gy the 5 year biochemical failure free survival rate was significantly worse at 51%.
Radiation: Dose and Delivery (EBRT)
Dose escalaton improves outcomes. The PROG 9509 trial was a proton trial comparing 70.2 GyE v. 79.2 GyE at 1.8 GyE/fraction. Zeitman reported in the JCO 2010 as follows:
- Phase III trial enrolling 392 men with low/low-intermediate risk prostate cancer (T1b-T2b PSA ≤ 15 ng/ml)
- All patients treated to 50.4 Gy at 1.8 Gy/fraction to the prostate and seminal vesicles using 10 - 23 MV photons 10 mm margin,
- Patients were then randomized to boost to prostate alone with a 5 mm margin in 11 or 16 fractions with protons
- ARM I: 19.8 CGyE in 11 fractions (Total dose 70.2 GyE)
- ARM II: 28.8 GyE in 16 fractions (Total dose 79.8 GyE
- Study end point was failure to achieve a complete response, positive biopsy, clinical evidence of progression or a PSA > 1 ng/ml more than 2 years after completion of radiation.
- Biochemical failure was determined by the ASTRO Consensus definition of three successive increases in PSA with failure date backdated to a point halfway between teh first increase and the PSA nadir or the initiation of salvage therapy.
- Biochemical failure was also determined by the Phoenix Consensus defined as a PSA value ≥ PSA nadir + 2 ng/mL after radiation therapy.
- Pre-treatment patient characteristics included:
- PSA range 1.24 - 14.68
- Gleason Score 2 - 10: GS ≤ 6 (295 -- 75%); GS 7 ( 59 -- 15%), GS 8-10 (33 -- 8% )
- Findings: Dose escalation decreased local failure from 32.2% l to 16.7%.
MD Anderson (Kuban in 2008, IJROBP) did another dose escalation trial of similar design and enrollment. The found that dose escalation improved 8 year freedom from failure to 78% from 59%. This improvement was seen in low and high risk groups but not in the intermediate subset. The 8 year cause specific survival was not significantly different at 99% v. 95% and the 8 year overall surival was also similar. This study showed a similar halving of the local failure rates as did Zeitman.
- Enrolled 301 patients with cT1b - T3 prostate cancer
- 21% low risk, 47% intermediate risk, 32% high risk
- Randomized to 70 Gy v. 78 Gy.
- Dose escalation improved 8 year freedom from failure 78% v. 59%
- The BFF improvement was seen in low risk and high risk groups but not intermediate risk group
- The 8 year cause specific survival was not different (99% v. 95%)
- The 8 year overall survival was not different at 78% v,. 79%
Patients are set up and planned using CT imaging. Treatment is generally delivered in the supine position with immobilization (α-cradles or vac-lok bags). Some institutions use a pelvic MRI or a urethrogram to locate the urogenital diaphragm to determine hte apex of the prostate. In our institution, we recommend a full bladder and an empty rectum. In cases where the rectum is full, we have the patient empty the rectum and refill the bladder prior to simulation, as we have found using CT and tomographic portal verification that the fullness of the rectum changes not only the position of the prostate but the shape of the prostate as well.
Treatment is delivered under image guidance, preferably with fiducial markers. There are several techniques used to verify the position of the prostate:
- 2D image guidance ± fiducial markers
- 3D image guidance ± fiducial markers
- Implantable RF transponder
- BAT Ultrasound localization
- Tomographic CT (conebeam CT) localization
Organs at Risk: Dose volume limits
Organs at risk include the rectum and bladder. The RTOG consensus statement regarding dose constraints at 1.8 Gy/fraction are:
For rectum:
- V70 ≤ 20% (EQD2 69 Gy (early) or 67.4 Gy (late)
- V50 ≤ 50% (EQD2 49.6 Gy (early) or 48.4 (late)
For Bladder:
- V70 ≤ 30%
- V55 ≤ 50%
Toxicity of Treatment
Surgery
The most common and significant post-prostatectomy side effects are
Nerve sparing surgery has improved the rate of erectilel function in 50% or more of men undergoing radical prostatectomy. Approximately 33% of men undergoing radical prostatectomy will have significant post-operative urinary incontinence according to Gunderson (Clin. Radiation Oncology 6th Ed. 2007). The level of incontinence peaks in the immediate post-operative period and improves over about a six month timeframe.
Radiation Therapy
The most common side effects of radiation therapy include:
(gradual onset over time about 50% who were previously potent will lose functional erections > 2 years post radiation.)
The rate of Grade 3 (RTOG) or higher late genito-urinary or gastro-intestinal toxicities with intensity modulated (IMRT) radiation therapy for prostate cancer is rare at ≤ 1%.
Intermediate/High Risk Prostate Cancer
According to the D'Amico criteria a prostate cancer is considered intermediate risk if he has any of the following factors (and no high risk factors):
- Stage T2b
- Gleason Score 7
- pre-treatment PSA 10 - 20 ng/ml
A patient by the D'Amico criteria has high risk prostate cancers if he has any or all of the following features:
- Stage ≥ T2c (T2c: involving both lobes of the prostate)
- Gleason Score ≥ 8
- PSA > 20 ng/ml
High Risk Prostate Cancer Treatment Options
Treatment options for intermediate risk prostate cancer include the following:
- External Beam Radiation therapy with short term androgen deprivation therapy (6 months) ± brachytherapy
- Brachytherapy ± androgen deprivation therapy
- Prostatectomy (less than ideal for patients with > 1 intermediate risk factor
- Active surveillance can be considered if life expectancy is < 8 - 10 years.
Surgery: Radical Prostatectomy
When stratified into intermediate and high risk prostate cancer by the D'Amico criteria, after prostatectomy alone, the 5 year Biochemical Disease Free Survival (bDFS) is approximately 65% for intermediate risk prostate cancer. For high risk cancers this falls to 35%.
When analyzed by staging, in prostatectomy alone treatment the 10 year bDFS is about 62% for cT2b and 57% for ≥ cT2c disease.
When analyzed by Gleason Score, after prostatectomy alone, bDFS at 10 years is 60% with Gleason score 7 (3 + 4) disease and drops to 33% with Gleason 7 (4+3) disease.
For Gleason 8 - 10 disease, the 10 year bDFS is 29%.
PSA is a third d'Amico determinant of risk. Stratification by PSA for post-prostatectomy patients with pre-treatment PSA of 10 - 20 ng/ml is 57% and for PSA > 20 ng/ml is 48%.
Of these three prognostic determinants, by far is the Gleason Score and nearly as importantly, the Gleason grade of the primary lesion.
Androgen deprivation therapy is indicated in post-prostatectomy patients that have positive regional lymph nodes. An improvement in survival has been reported in 2006. Messing in the Lancet established androgen suppression therapy after prostatectomy in this setting and demonstrated improved survival. Median survival improved from 11.3 years to 13.9 years. The main criticism of this study was that androgen suppression was not initiated in the observation arm until clinical evideice of progression rather than biochemical evidence of progression was noted.
Neoadjuvant (pre-operative) androgen deprivation therapy has been studied. Despite improvement in pathologic features of prostatectomy, long term bFFS rates do not appear to be improved. This negative result has been confirmed by a number of randomized studies. The benefits of preoperative androgen deprivation therapy include decreased positive margins and decreased positive lymph nodes. Longer duration is associated with greater improvement.
LDR Brachytherapy
The American Brachytherapy Society guidelines state that LDR brachytherapy alone for high risk prostate cancers is not appropriate. It may be used in conjunction with external beam (combination therapy) and in very carefully selected patients with intermediate risk disease. This might include low volume disease Gleason 7 (3+ 4) but not (4+3). d'Amico retrospectively reviewed LDR brachytherapy alone and found worse biochemical progression free survival at 5 years. compared to prostatectomy and EBRT. Several single institution series contradict this study and suggest in well selected intermdeiate risk patients receiving a high quality implant, excellent outcomes are found. (Roach/UCSF)
The American Brachytherapy Society guidelines allow neoadjuvant androgen deprivation therapy to cytoreduce large prostate glands (Nag, IJROBP, 1999), but several lare retrospective studies failed to demonstrate NAT improves cancer control with LDR brachytherapy.
Combined External Beam and Brachytherapy without androgen deprivation (NAT) therapy is not commonly used with high risk disease. Multiple institutional studies have shown good long sterm outcomes in intermediate risk patients with bPFS10 of 79% - 90%.
There has been one randomized controlled trial of EBRT ± brachytherapy boost in patients with intermediate and high risk disease (Sathya JCO 2005). The details of this study are:
- EBRT alone to 66 Gy compared to EBRT to 40 Gy followed by 192Ir 35 Gy over 48 hours plus 40 Gy EBRT.
- Sathya found improved bFFS and clinical FFS rates of 29% (66 Gy alone) compared with 51% (40 Gy + 35 Gy implant)
- This study suffers from a relatively low dose in the control arm and the lack of ADT.
External Beam plus HDR Brachytherapy
EBRT + HDR is not commonly used in intermediate and high risk prostate cancer patients. Long term data from Demanes and others have shown good long-term results in both intermediate and high risk patients. These studies have shown bPFS10 of 87% in intermediate risk and 63% in high risk patients.
There has been one randomized controlled study of EBRT ± HDR boost which included low, intermediate and high risk patients. (Hoskin 2007, Radioth Oncol.) The study characteristics are:
- Arm I: EBRT to 37.75 Gy at 2.75 Gy/fraction (EQD2=41.1) + HDR 17 Gy at 8.5 Gy fractions
- Arm II: EBRT alone to 55 Gy at 2.75 Gy/fraction (EQD2 = 63.3 Gy)
- Found improved biochemical RFS of 5.1 years median survival with HDR, over 4.3 years without.
- Toxicity was no different in the two arms.
- Most patients had NAH/ADT in both arms.
- Again, the major shortcoming of this study was the low dose in the control arm and the lack of control for ADT.
Androgen Deprivation Therapy
There have been several studies which support the use of androgen deprivation therapy in locally advanced rpxtate cancer. RTOG 8610 was the first of these studies which included high risk prostate cancer by the d'Amico criteria. To date, no study published has looked exclusively at intermediate risk prostate cancer. RTOG 9408 enrolled mainly intermediate risk groups, but also enrolled all risk groups. This study found a 12 year overall survival benefit with the addition of short course hormonal therapy.
D'Amico, Laverdiere and Dehham in TROG 96.01 all showed improved disease specific outcomes with the addition of short course androgen deprivation therapy with external beam radiation therapy in intermediate risk patients.
RTOG 8610: Short Course Androgen Deprivation Therapy (Roach, 2008 JCO). RTOG 8610 enorlled 456 men with cT2-T4(bulky disease). N1 patients were eligible if nodal disease was below the common iliac. All were treated with EBRT 65 - 70 Gy and randomized to 4 months of androgen suppression therapy beginning 2 months prior to EBRT or observation with androgen deprivation at relapse (ADT). Finding:
- 456 enrolled with cT2-T4(bulky disease); N1 if below common iliac
- All treated with EBRT 65 - 70 Gy and randomized to 4 months of ADT starting 2 months prior to EBRT or observation with ADT at relapse
- 10 year overall survival and median survival favored short term NAH/ADT 44% and 8.7 years over no NAH/ADT 34% and 7.3 years, but the difference was not statistically significant.
- Short course ADT improved the 10 year cause specific mortality 23% to 36% without hormonal therapy
- Short course ADT improved distant metastases 35% over RT alone 47%.
RTOG 9408 Short Course Neoadjuvant Androgen Deprivation Therapy This study enrolled 2028 patients with T1b-T2b, PSA ≤ 20 ng/ml prostate cancer. Patients were randomized to EBRT alone to a dose of 68.4 Gy ± 4 months of ADT, starting 2 months prior to prostate radiation. 12 year overall survival favored short course androgen deprivation therapy arm 56% over no hormones 51%.
TROG 96.01 Length of NAH/ADT Starting 2 Months prior to EBRT (Denham Lancet 2005).This trial enrolled 818 patients with T2b-T4 prostate cancer treated with EBRT to 66 Gy at 2 Gy/fraction As follows:
- Randomized 818 patients with T2b-T4 prostate cancer to 0, 3 or 6 months androgen deprivation therapy starting 2 months prior to radiation.
- With a median follow up of 5.9 years, the 3 and 6 month ADT arms had improved
- local failure rates
- improved biochemical failure rates
- improved freedom from salvage treatmen
- The six month arm also had improved distant failure and prostate cancer specific survival compared with the radiation alone arm
- There was no overall difference in survival and no consistent cancer control difference between 3 and 6 month arms.
D'Amico DFCI Trial: Benefit of Short Course ADT in locally advanced prostate cancer (JAMA 2008).The DFCI trial enrolled 206 men with cT1b - T2b prostate cancer with one of the following characteristics: PSA 10 - 40 ng/ml, Gleason Score 7 - 10, or ECE/seminal vesicle invasion by MRI study. Patients were randomized to EBRT to 70 Gy ± 6 months of ADT starting 2 months prior to EBRT. The ADT arm improved 8 year overall survival from 61% (no ADT) to 74%.An unplanned subset analysis suggested that the benefit of hormone therapy may be limited to men without significant comorbidities.
Hormonal therapy in patients being treated with EBRT and ADT is usually started 2 months prior to the start of radiation therapy. Pre-clinical experiments suggest that neoadjuvant hormone therapy (NAH) may improve prostate cancer radiation sensitivit compared to concurrent ADT. This may be due to improved tumor oxygenation with neoadjuvant androgen suppression.. Randomized trials above (RTOG 8610, DFCI d'Amico Trial, TROG 96.01 all demonstrating the advantage of EBRT combined with ADT all had ADT starting 2 months prior to the start of EBRT. However, RTOG 9413 compared neoadjuvant/concurrent ADT to short course adjuvant therapy showed no benefit (bPFS) to neoadjuvant ADT.
RTOG 9413: Timing of ADT in Intermediate and High Risk Prostate Cancer Patients (Lawton UJROBP 2007) RTOG 9413 compared the timing of ADT in intermediate and high risk prostate cancer patients to 4 months of ADT starting 2 months prior to or immediately following radiation therapy. This study was a 2 x 2 factorial desing. It randomized 1323 patients as follows:
- Randomization 1: ADT starting to months prior to RT or starting immediately following RT
- Randomization 2: RT field size was randomized to whole pelvis (WP) or to prostate and seminal vesicles only (PSVO)
- After a median follow up of 7 years, there was no difference in PFS in neoadjuvant v. adjuvant arms and no difference in PFS in the WP or PSVO arms.
- Interpretation is limited by an unexpected interaction between the two randomizations
RTOG 9910 Duration of neoadjuvant HT -- results not in yet. The duration of ADT prior to EBRT is traditionally 2 months. There is only one published randomized controlled study examining the optimal duration and timing of NAH in localized prostate cancer (Crook, 2009, IJROBP). This trial enrolled 378 men with localized prostate cancer of any risk group. All were treated with EBRT to 66 - 67 Gy without concurrent ADT. Patients were randomized to 3 months v. 8 months of NAH. The 5 year Freedom From Failure (FFF) did not differ between the treatment arms. An unplanned subgroup analysis demonstrated the 5 year disease free survival was improved for high risk patients at 71% for longer term therapy as opposed to 42% for short term therapy.
RTOG 9910 evaluated 2 months or 7 months of NAH but results are not yet available.
Long term androgen deprivation therapy has been extensively studied by a number of trials, including the Casodex Early Prostate Cancer Trial, EORTC 22863, RTOG 9202, and EORTC 22961. These studies have shown the benefit of long term androgen deprivation on overall survival after EBRT. The first trial to demonstrate improved overall survival is RTOG 8531.
RTOG 8531: Long Term Androgen deprivation therapy. (Pilepich MV UJROBP 2005)This study enrolled 945 men with cT3 non bulky disease after prostatectomy or N1 disease. Patients were treated with EBRT to a total dose of 65 - 70 Gy or post-operatively to 60 - 65 Gy. Following radiation (definitive or adjuvant) and randomized to indefinite duration androgen therapy or observation.
- 945 men with cT3, pT3 (s/p prostatectomy) or N1 disease
- All received post prostatectomy RT to a total dose of 65 - 70 Gy (definitive) or 60 - 65 Gy (post op)
- Randomized: ADT indefinitely or observation
- ADT improved 10 year OS 49% from 39%
- ADT improved 10 year cause specific mortality
- ADT improved 10 year local failure to 23% from 38%
- ADT improved 10 year distant failure rates to 24% from 39%
- On subset analysis benefits were limited to Gleason Scores ≥ 7 and were especially important in GS 8 - 10
The Casodex Early Prosate Cancer Trial (Wa, J. Cancer Res, Clin, Oncol 2006).This trial randomized 8113 men to observation of long term Casodex after local therapy. Local therapy consisted of radiation or prostatectomy, Casodex was continued for 2 years or until progression of disease. In the radiation therapy subgroup, 1730 cases, after a median follow up of 7.2 years, adjuvant long term Casodex did not result in better overall survival or pathologic cause specific survival.
In an unplanned analysis, a subgroup of locally advanced patients with T3-4 or N1 disease did demonstrate an overall survival and pathologic cause specific survival improvement.
EORTC 22863 -- The Bolla Trial Lancet 2002. This is the classic trial designed to study the benefits of long term androgen deprivation therapy found long term androgen deprivation therapy improved 5 year overall survival to 78% from 62%, cause specific survival to 94% from 79%, reduced local failure to 1.7% from 16.4% and reduced distant failure to 9.2% from 29.2%
- Studied 412 men with cT3-T4 disease of any grade or cT1-T2 WHO Grade 3.
- All received 70 Gy EBRT
- Randomized: 3 years androgen suppression concurrent with EBRT or observation with androgen suppression at relapse.
- Long term androgen suppression improved OS-5 to 78% from 62%
- Long term androgen suppression improved CSS to 94% from 79%
- Long term androgen suppression decreased local failure to 1.7% from 16.4%
- Long term androgent suppression decreased distant failure to 9.8% from 29.2%
RTOG 9202 Studied the benefit of long term androgen suppression in locally advanced prostate cancer (Horwits JCO 2008)This study enrolled 1541 men with cT2c - T4, PSA < 150 ng/ml prostate cancer. It found no improvement in survival in the entire cohort, but there was improved 10 year cause specific survival, local progression control, and distant metastases. An unplanned subgroup analysis in patients with Gleason ≥ 8, demonstrated improved overall survival in the immediate androgen suppression group.
- Enrolled 1541 men with cT2c - T4, PSA < 150 ng/ml
- All were treated with 2 months androgen suppression followed by concurrent androgen suppression and EBRT to 65 - 70 Gy.
- Randomized to an additional 2 years of androgen suppression or observation with androgen suppression at relapse.
- Long term androgen suppression was not associated with improved overall survival in the entire cohort, but was associated with improved OS in unplanned subgroup analysis of Gleason ≥ 8 disease.
- LT-AS was associated with improved cause specific survival at 10 years to 89% from 84%
- LT-AS was associated iwth improved local progression to 12% from 22%
- LT-AS was associated with better distant metastases failure rate to 15% from 23%.
- LT-AS on unplanned subgroup analysis demonstrated improved OS-10y in Gleason Score 8 - 10 disease to 45% from 32%.
EORTC 22961 (Bolla NEJM 2009) Compares short course AS therapy to long course AS therapy. Bolla enrolled 1113 men with cT2c-T4/N0 or cT1c-T2b N1-N2 prostate cancer and randomized to six months or 3 years of neoadjuvant, concurrent and adjuvant androgen suppression. Bolla found that men recieving 3 years AS had better OS and cause specfic mortality than short term androgen suppression therapy. He reported that the long term overall quality of life did not differ between the two arms
- EORTC 22961 Long Term v. Short Term neoadjuvant/concomitant/adjuvant AST
- Enrolled 1113 men with cT2c-T4 N0 or cT1c-T2b N1-N2 prostate cancer
- All were treated with 70 Gy radiation and then randomized.
- Randomization: 6 months AST or 3 years AST.
- Men receiving 3 years of AS had superior OS (85%) over short term treatment (81%)
- LT AST men had superior cause specific mortality at 5 years (3.2% compared with 4.7%)
- Quality of Life did not differ appreciably between the studies.
Taken together these studies, in particular RTOG 9202 and EORTC 22961 suggest long term (2-3 years) androgen suppression is superior to short course andorgen suppression in high risk prostate cancer. The optimum duration of androgen suppression is not yet well determined.
Radiation Therapy Treatment in Intermediate-High Risk Prostate Cancer
Dose
Radiation doses in intermediate and high risk prostate cancer who do not receive androgen suppression therapy should be ≥ 74 Gy in 2 Gy fractions. There have been four EBRT dose escalation studies including intermediate and high risk patients. These studies include:
- MDACC Dose Escalation Trial
- PROG 95.05 Trial
- Dutch Dose Escalation Trial
- MRC RT01 Trial
All four of these trials have demonstrated at least improved biochemical evidence of improved control with dose-escalated EBRT. The role of high dose EBRT is less clear in the setting of androgen suppression therapy. The Dutch Dose escalation trial allowed androgen suppression but only a monorit of men received it. (Peeters JCO 2006). The MRC RT01 trial mandated neoadjuvant and concomitant androgen suppression. The 5 year outcomes favored dose escalation.
MDACC Dose Escalation Trial (Kuban 2008 IJROBP) Kuban enrolled 301 patients with cT1b-T3 prostate cancer. None were treated with androgen suppression. 21% were low risk, 47% were intermediate risk and 32% were high risk. Patients were randomized to 70 Gy or 78 Gy. Dose escalation improved 8 year freedom from failure to 78% from 59%. This improvement was seen in low and high risk groups but not in the intermediate risk groups. The 8 year cause specific survival was not significantly different at 99% compared with 95% nor was the 8 year overall survival different at 78% and 79%.
The PROG 95.09 RCT on Dose Escalation (Zeitman JAMA 2005) This trial enrolled 393 patients with T1b-T2b PSA < 15 ng/ml prostate cancer. Patients were randomized to 70.2 Gy or 79.2 Gy radiation therapy. Reduced field boost treatment to the prostate only was given by protons with 50.4 Gy delivered to the prostate and seminal vesicles by photon radiotherapy. Dose escalation improved 5 year freedom from biochemical failure to 80% from 61% and five year local control to 55% from 48%.
An unplanned subgroup analysis showed a significant improvement in freedom from biochemical failure in both low and intermediate risk subsets.
MRC RT01 RCT Studied the benefit of dose escalation with neoadjuvant/concurrent AS (Dearnaley 2007 Lancet) The Medical Research Council of Great Britain enrolled 843 men with cT1b - T3a, PSA < 50 ng/ml in the RT01 trial. These men were all treated with androgen suppression 3-6 months neoadjuvant and concurrent androgen suppression. These men were then randomized to EBRT to 64 Gy or 74 Gy. The dose escalation improved bPFS-5yr to 71% from 60%. Local control, freedom from salvage AS and distant metastases free survival favored the dose escalation arm. The endpoints for DMFS, LC and FF salvage were not statistically significant
SPCG-7: Role of Androgen Suppression Alone in localized high risk cancer patients who cannot tolerate local management or have short life expectancy. Androgen suppression alone for localized high risk prostate cancer may be considered for men who cannot tolerate other treatment (radiotherapy) or for those who have a short life expectancy (defined as < 5 years). SPCG-7 demonstrated that the addtion to EBRT to long term Androgen suppression conferred a survival advantage in high risk men.
The Scandinavian Prostate Cancer Group Study, SPCG-7 enrolled 875 men with cT1b-T2 NO WHO Grade 2-3 or cT3 of any grade, node negative disease. All men were treated with androgen blockade for 3 months followed by anti-androgen alone (flutamide). Androgen blockade was continued indefinitely. Patients were randomized to 70 Gy EBRT starting after 3 months of anti-androgen treatment or no local therapy. At median follow up of 7.6 years, the addition of EBRT improved OS-10 to 70% from 61% and CSS-10 to 88% from 76%. EBRT reduced the 10 year prostate cancer specific mortality in half to 12% from 24%. (CSS-10 and OS-10 refer to cause specific and overall survival at 10 years.)
Node Positive Prostate Cancer
There has been no definitive study to assess the role of local/regional radiation therapy in node positive prostate cancer. There has been a retrospective review by Zagars in 2001 which looked at this issue. Zagers suggested that EBRT added to long term androgen suppression may confer an overall survival benefit to node positive patients. In addtion, a subset analysis from RTOG 8531(Lawton, 2005 JCO) suggests that long term androgen suppression with EBRT confers an overall sruvival benefit compared with EBRT alone in node positive patients. The long term biochemical control in node positive patients remains poor (PSA < 1.5 ng/ml).
Three studies: RTOG 7706, RTOG 9413 and GETUG-01 have examined the role of pelvic nodal irradiation in node. None showed a cancer control benefit to irradiation of the pelvic nodes. All of these trial included men who may have been at low risk for harboring nodal disease. Pelvic nodal radiation may be useful in men at very high risk of having nodal disease. Treating nodes in prostate cancer is controversial.
Toxicity of Treatment
Treatment toxicity in intermediate and high risk prostate cancer is similar to that of low risk prostate cancer. The most common acute side effects of prostatic radiation therapy include:
- fatigue
- urinary urgency or frequency
- proctitis/diarrhea or loose stools
The most common late/chronic affects of radiation include:
- erectile dysfunction
- cystitis
- proctitis /loose stools and bleeding
Numerous studies indicate the level of late Grade 3 or higher GU/GI toxicity in radiation for prostate cancer is rare at ≤ 1%. The rate of erectile dysfunction is considerably less rare at 50% of men who were previously potent will no longer be able to maintain an erection at > 2 years from radiation. Androgen deprivation/suppression does not appear to affect the GU/GI toxicity of radiation therapy. Multiple studies have shown no effects.
Androgen suppression therapy has significant short term side effects in most men: hot flashes, decreased/absent libido, and fatigue. Long term side effects include gynecomastia, anema, decreased muscle mass, decreased bone mineral density, obesity, mood changes/swings, dyslipidemia, insulin resistance, possibly, coronary artery disease or diabetes (metabolic syndrome). 50% of men treated with bicalutamide will experience breast tenderness and gynecomastia.