Pancreatic Adenocarcinoma
Epidemiology and Demographics
There are bout 30,000 cases of pancreatic cancer per year in the United States. The disease is highly fatal and is the 10th most common cancer diagnosis but the 4th most common cause of cancer death in the U.S. The incidence is similar among men and women, but there is a predilection toward African descent. The peak age of incidence of pancreatic cancers is in the 6th - 7th decades.
Environment, Natural History and Genetics
The most common environmental risk factors for pancreatic cancers include:
- Tobacco smoking
- 2-naphthylamine
- benzidine
Approximately 5% of pancreatic cancers are familial. Genetic mutations of p16 and BRCA2 are the most common familial genetic abnormalities. The K-ras oncogene is present in about 85% of all pancreatic cancers.
Chronic pancreatitis is not associated with the risk of pancreatic adenocarcinoma. Commonly pancreatic adenocarcinoma arises in the head of the pancreas, next in the body and least commonly in the tail.
- 75% Head of Pancreas
- 15% Body of Pancreas
- 10% Tail of Pancreas
About half of all pancreatic cancers have distant metastases at diagnosis. About 25% have regional nodal metastases at diagnosis. The most common sites of pancreatic cancer metastases include liver, peritoneal surface and lungs.
Periampullary tumors arise frm the ampulla of Vater, the distal common bile duct r the adjacent duodenum.
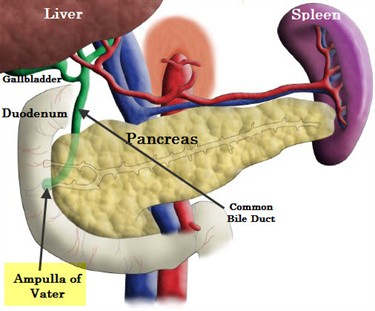 |
There is no proven role for screening pancreatic cancers There are studies evaluating EUS roles but no proven benefit.
Nearly all pancreatic cancers are exocrine (95%). There are four common pathologic subtypes of adenocarcinoma of the pancreas:
- Ductal adenocarcinoma (40%)
- Mucinous cystadenocarcinoma
- Acinar cell carcinoma
- Adenosquamous carcinoma
The primary presenting signs of pancreatic adenocarcinoma are pancreatic or bilary duct obstruction, jaundice and abdominal pain.
Workup and Staging
The differential diagnosis of a pancreatic mass includes
- exocrine cancer
- islet cell /neuroendocrine cancer
- cystic adenomas
- intraductal papillary carcinomas
- lymphoma
- acinar cell carcinoma
- metastatic carcinomas
The general workup is H&P, CBC, CMP, CA 19-9, tri-phasic thin sliced CT of the abdomen, chest imaging ± ERCP or EUS with FNA for diagnosis and possibly, stent placement. Patients with Lewis Antigen AB negative cannot secrete CA 19-9. This phenotype is present in about 5 - 10% of the population.
Tissue diagnosis is obtained from ERCP, endoscopic ultrasound guided fine needle aspiration, CT guided FNA or pancreatic resection. Histologic diagnosis is not necessary before resection of a pancreatic mass. ERCP and endoscopic resection (2%) is associated with a lower risk of seeding the peritoneum than CT guided FNA (26%).
CA 19-9
CA 19-9 has been reported as a prognostic indicator. The RTOG 9704 study enrolled 53 patients (14%) with CA 19-9 > 90 U/ml. Only 2 of these patients survived up to 3 years.
Staging and Classification
Pancreatic cancer is a surgical disease with roles for adjuvant and definitive chemotherapy and radiation therapy. The NCCN 2010 classified pancreatic cancers as :
- Resectable (T1-T3 N0-1) AJCC Stage Group I-II
- Borderline Resectable (T4N0-1) AJCC Stage Group III
- Locally advanced (T4 N0-1) Stage III
- Metastatic (M1) Stage IV
The NCCN criteria for resectable pancreatic adenocarcinoma are:
- Patent superior mesenteric vein/portal vein confluence
- Clear fat planes around the SMA (superior mesenteric artery) confluence
- No nodal metatastases or other mets beyond the field of resection.
The NCCN defines borderline resectability as
- Severe unilateral SMV/portal confluence impingement
- Tumor abutting the SMA
- Gastroduodenal artery encasement up to the hepatic artery
- Limited involvement of the IVC
- Short segment SMV occulsion with patent vein both proximally and distally
- Colon or mesocolon invasion
 |
For pancreatic tail lesions, borderline resectable lesions refer to lesions that invade the adrenal gland, colon, mesocolon or kidney. Pancreatic head lesions are frequently more resectable because they become symptomatic earlier in the disease.
Over 80% of pancreatic cancers deemed resectable by CT are resectable at surgery.
AJCC Staging
| T1 | limited to the pancreas ≤ 2 cm |
| T2 | limited to pancreas > 2 cm |
| T3 | extends beyond pancreas but without celiac or SMA involvement |
| T4 | celiac axis or SMA involvement |
| N0 | No nodal metastases |
| N1 | Nodal metastases |
| T | N0 | N1 | M1 |
| T1 | IA | IIB | IV |
| T2 | IB | IIB | IV |
| T3 | IIA | IIB | IV |
| T4 | III | III | IV |
Positive washings at them time of laparoscopy are staged Stage IV (M1).
The AJCC has established a separate staging system for Ampulla of Vater, distal CBD and duodenal carcinomas.
| T | Ampulla of Vater | Distal Common Bile Duct | Perihilar Bile Ducts |
| T1 | Limited to Amuplla of Vater or sphincter of Oddi | Confined to Bile Duct histologically | Confined to bile duct with extension up to muscle layer or fibrous tissue |
| T2a | Invades duodenal wall | Invades beyond wall of bile duct | Invades wall to surrounding adipose tissue |
| T2b | (T2 Only) | (T2 Only) | invades adjacent hepatic parenchma |
| T3 | Invades pancreas | Invades gall bladder, pancreas, duodenum or other adjancet organs without celiac axis or SMA involvement | invades unilateral branches of teh portal vein or hepatic artery |
| T4 | Invades peripancreatic soft tissues or other adjacent organs or structures other than pancreas | Involves celiac axis, or SMA | invades main portal vein or its branches bilaterally; or the common hepatic artery or the second order bilary radicals bilateraly |
Treatment and Prognosis
Surgery is the primary treatment. Surgery used in head of the pancreas lesions (75% of all cases) involves a classic Whipple procedure. The Whipple procedure is a pancreaticoduodenectomy. Alternatively, a pyloris preserving pancreaticoduodenectomy may be performed.
Whipple Procedure
The Whipple procedure involves resecting the pancreas with three anastomoses:
- pancreaticojejunostomy
- choledochojejunostomy (hepaticojejunostomy)
- gastrojejunostomy
Post operative favorable prognostic indicators include:
- R0 (negative margin) resection
- Low grade (G1)
- Small tumor size (T1, small T2 lesions ≤ 3 cm)
- N0 (node negative disease)
Surgery is only beneficial if there is a complete resection (R0). Retrospective evidence suggests that survival is similar with patients who have R1 (microscopic residual) and R2 (gross residual) disease and those who have definitive chemo-radiation therapy. Planned resection should be performed in patients where complete resections (R0) resections are likely. Debulking surgery does not improve outcome over definitive chemo-radiation therapy. Autopsy findings in in 78 pancreatic adenocarcinoma patients demonstrate local recurrence in 78% was a component of failure and hepatic recurrence in 61.5%.
There has been no demonstrated survival benefit demonstrated in performing and extended lymphadenectomy. Resectable patients should not undergo extended retroperitoneal lymphadenectomy. Riall in 2005 published a randomized controlled trial demonstrating no statistically significant differences in survival at 25% and 31% 5 year survival.
Where possible, surgery remains the first line treatment of pancreatic adenocarcinoma. The Japanese Pancreatic Adenocarcinoma Study Group compared surgery alone against definitive chemo-radiation therapy in a randomized controlled trial. This trial, published in 2008 was stopped early due to clear benefit of surgery. Median survival was 12 months in the surgery arm and 9 months in the chemo-radiation arm. Radiation therapy was delivered to 50.4 Gy with ci5FU. Not only was the median survival much worse in the chemo-radiation arm, but the 5 year survival in the chemo-radiation arm was dismal. Surgical 5 year survival rates were not good, but at 10% were better than 0%.
Post resection adjuvant treatment options after pancreaticoduodenectomy include
- Adjuvant gemcitabine (CONKO-1 Trial)
- Adjuvant gemcitabine alone → 5FU + Radiation → gemcitabine alone (RTOG 9704)
- Adjuvant 5FU/Radiation (GITSG 91-73) → consider maintenance gemcitabine
- Adjuvant 5FU → 5FU+radiation → 5FU (RTOG 9704)
- Observation alone
Post Operative Adjuvant Therapy
Standard and total dose/fractionation after surgical resection uses a dose to regional lymphatics and tumor bed of 45 Gy with a boost to the tumor bed of between 50.4 Gy and 54 Gy (boost 5.4 Gy - 9 Gy). All doses are given at 1.8 Gy/fraction. The standard radiotherapy fields are tumor bed and at-risk regional lymph nodes. Add 1 -2 cm margins for motion and setup error. The boost fields is the tumor bed plus margin.
Studies Favoring Post-operative Chemotherapy and Radiation Therapy
GITSG 91-73 Post-op observation compared to Post-Op radiation with Split Course Radiation (Kalser 1985 Arch. Surg) GITSG 9173 Trial first reported a benefit to adjuvant post-operative concurrent chemotherapy with radiation therapy in 1985. The trial information is:
- 43 patients with resectable pancreatic cancer were randomized to Post-op observation or Chemotherapy and radiation therapy using split course radiation therapy
- Radiation was delivered: 40 Gy with a 2 week break after 20 Gy with intermittent bolus 5FU. → 2 full years of adjuvant 5FU alone
- Adjuvant Chemo-radiation improved median survival to 20 months from 11 months (observation arm)
- Adjuvant chemo-RT improved OS2 to 43% from 18% and OS5 to 18% from 5%
Most radiation oncologists do not believe that split course radiation is as effective as continuous course treatment.
Mayo Clinic (Corsini 2008) Retrospective review of resectable T1-T3 N0 patients who received adjuvant chemotherapy/radiotherapy or observation.
This study used a more conventional continuous course radiation therapy treatment at 1.8 Gy to 50.4 Gy. 98% of all patients received continuous infusion 5FU with radiation. This cohort was compared with those who were observed with no further treatment. The study showed that chemo-radiation therapy improved median survival, and two and five year overall survival:
- Median survival improved to 25.2 months from 19.2 months
- OS2 improved from 39% to 50%
- OS5 improved from 17% to 34%
Johns Hopkins 2008 Retrospective review of Post-operative Adjuvant Chemo-radiation v. Observation (Herman 2008)
This study looked at 616 patients who were treated with pancreaticoduodenectomy who recieved RT/5FU or observation. Chemotherapy was 5FU based. The study demonstrated improved median survival at 21 months in the treated cohort compared with 14 months in the observed cohort.
- Improved median survival with chemo/RT 21 months from 14 months (obs. alone).
- Improved OS2 44% with chemo/RT over 32% without chemoRT
- Improved OS5 20% with chemo/RT over 15% with observation alone.
SEER Review of 3008 patients receiving re-operative or post-operative radiation therapy or no adjuvant treatment. (Hazard 2007)
This study compared cohorts who either did or did not get radiation therapy treatments. It reported improved survival in patients who had direct extension beyond the pancreas or in node positive patients with adjuvant radiation therapy. .
- 3008 Patients with resected pancreas were examined in this retrospective review
- 1228 patients received radiation therapy either pre-operative or post-operative, only 23 had pre-operative RT
- Patients receiving RT had improved median survival at 21 months from 14 months.
- Improved OS5 at 13% compared with 9% in observation alone.
- Radiation therapy improved survival in those patients with node postive or direct extension beyond the pancreas but not in T1-2N0 paitients.
- Radiation improved Cause Specific Survival in patients with positive lymph nodes.
While not a part of this study, a re-analysis of the SEER data looking at pre-operative v. post-operative radiation therapy in showed improved overall survival in patients with pre-operative radiation therapy.
Johns Hopkins-Mayo Clinic Collaborative Study (Hsu 2009)
This study was another retrospective study of post-operative chemotherapy+radiation compared with observation alone. This study showed a median survival, 2 and 5 year overall survival improvement with chemo/radiation therapy over observation alone.The details:
- Restrospective study looking at post-operative pancreatic adenocarcinoma treated with adjuvant chemtherapy/radiaiton or surgery alone.
- Treatment arm was radiation therapy to 50.4 Gy at 1.8 Gy/fraction plus 5FU based concurrent chemotherapy
- Finding: Median survival improved to 21.1 months from 15.5 months with chemo/RT
- Finding: OS2 improved to 44% from 34.6%
- Finding: OS5 improved to 22.3% from 16.1%
- Adjuvant chemo-RT improved survival 33% when propensity score analysis was used and patients were matched by age, marings, nodes, and T-stage.
EORTC 40891 Similar to GITSG 91-73 except no post radiation 5FU (Klinkenbijl, 1999 Ann. Surg.) This European study supported the benefit of post-operative radiation therapy combined with 5FU. The details:
- enrolled T1-T2 N0-N1 pancreatic cancer or T1-T3 N0-N1 periampullary adenocarcinoma.
- Randomization: Observation alone or Chemo-radiation therapy using split course radiation therapy.
- RT: 40 Gy with 2 week break after 20 Gy with intermittent bolus 5FU. No additional chemotherapy after completion of radiation.
- Median survival and progression free survival difference was not statistically significant with Chemo-RT arm median progression free survival 17 months and observation arm m.PFS 16 months.
- Median survival was 24 months (chemoRT arm) compared with 19 months in the observation arm.
- For the pancreatic patients there was a trend toward improved 5 year survival at 20% in the CRT arm over 10% in the observation arm. p=0.09.
- While the authors concluded that post-operative chemo/RT was not warranted, a subsequent re-analysis of this study did demonstrate a significant survival benefit with adjuvant therapy (Garofalo 2006 Ann. Surg).
Studies Favoring Chemotherapy without Radiation Therapy
ESPAC-1 Four Arm Trial of Surgery ± CRT or ± Chemotherapy (Neoptolemos, 2001, Lancet) The ESPAC-1 Study enrolled patients with grossly resected pancreatic cancer. It was a 4 arm trial that compared ± post-operative chemotherapy/radiation therapy, and ± chemotherapy alone.
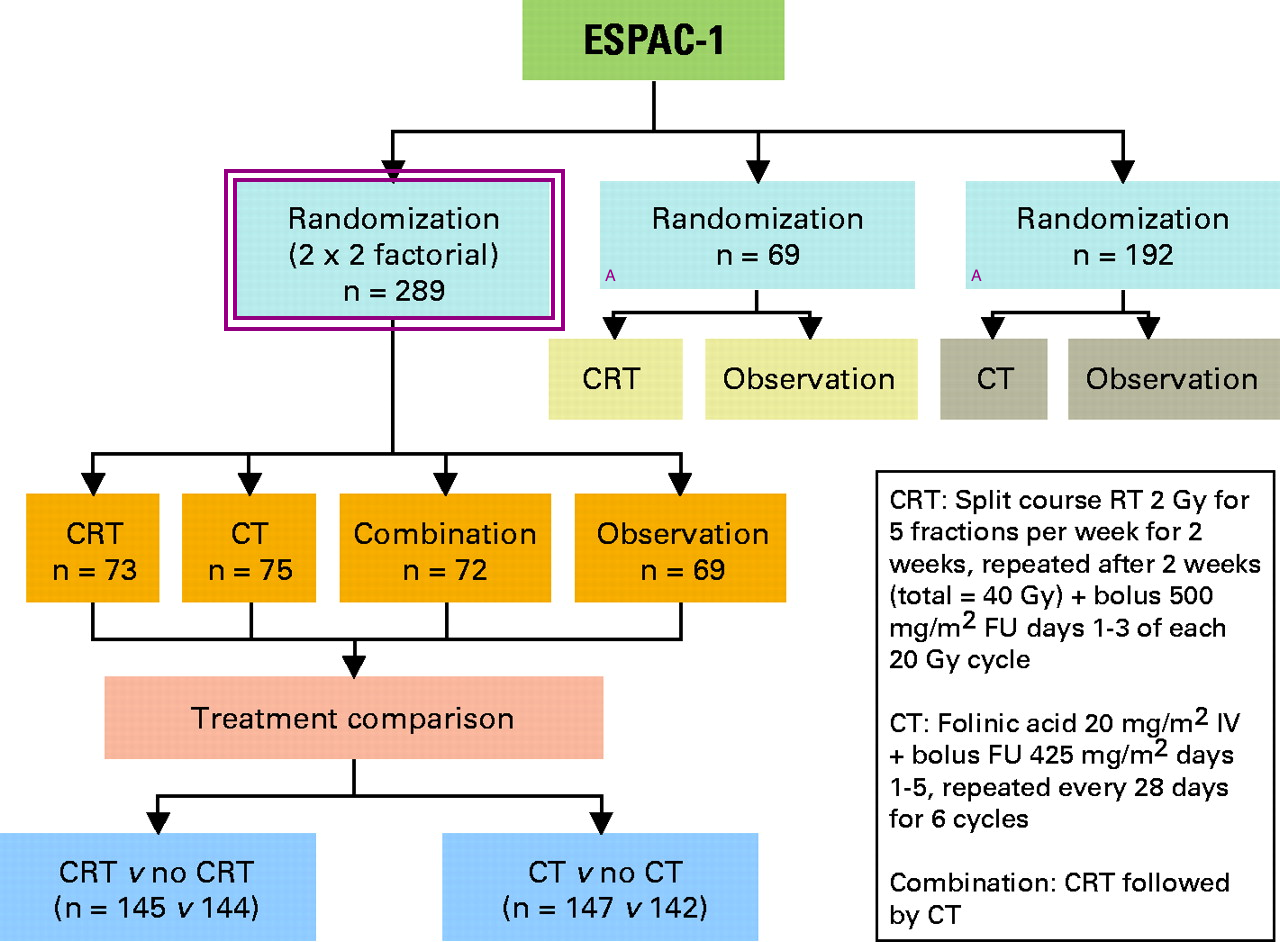 |
The study was intended to be a 2x2 factoral randomization comparing Chemotherapy+radiation therapy to chemotherapy to CRT followed by chemotherapy to observation. The study introduced two further separate randomizations: chemoradiotherapy or observation and chemotherapy or observation to allow the inclusion of patients when either clinician or patients were unwilling to accept the 2x2 randomization. The original study design is indicated with box and the newer arms are indicated with an A.
Here are the key features of the ESPAC 1 Trial:
- Enrolled 541 patients with resected pancreatic and peri-ampullary adenocarcinomas only 289 of which were randomized to the 2x2 intended arm.
- Trial arms:
- Chemo/RT: 40 Gy split course with 5FU (20 Gy → 2 weeks break → 20 Gy
- Adjuvant chemotherapy alone 5FU/leukovorin
- chemo/RT → additional chemotherapy
- post-operative observation alone.
- Note: There were two additional (non-randomized arms in the 2x2 factoral regions.
- Results:
- Contradictory when looking at randomized and non-randomized groups in initial analysis
- For all patients chemotherapy improved median survival to 19.7 months from 15.9 months.
- For randomized patients chemotherapy had no effect on median survival at 17.4 months and 15.9 months without chemotherapy.
- In the final analysis Neoptolemos concluded that chemotherapy was of benefit with OS5 of 21% compared with observation at 8% and chemotherapy/radiation therapy was detrimental with OS5 at 10% compared with 20% without radiotherapy.
Criticisms of this Study:
- There was no radiation therapy quality assurance.
- Only 128 patients had the details of the radiation therapy fields available.
- Of those 128 patients only 90 actually received the full prescribed dose.
- The radiation course was offered as a split-course which is inferior treatment
- Progressive disease precluded radiation in 19 patients.
- Physicians and patients were able to avoid the original randomization design and directly select non-randomized arms
- "Background treatment " was allowed: Patients in observation arms may have received chemotherapy and/or radiation therapy.
ESPAC Meta-analysis 2009 (Neoptolemas) examined 822 patients who received either adjuvant 5FU/folinic acid or observation after surgical resection. Adjuvant 5FU/FA improved median survival to 23.2 months from 16.8 months.
CONKO-001 Randomized study of Gemcitabine compared with observation alone (Oettle 2007).. This study was a randomized study of Gemcitabine for six cycles compared with observation alone in post-operative pancreatic cancer patients. It demonstrated, adjuvant gemcitabine improved disease free survival to 13.4 months from 6.9 months. No survival benefit was identified. The study excluded patients with a pre-operative CA 19.9 ≥ 2.5 x normal level
Studies of Different Chemotherapy/Radiation Regiments
RTOG 9704/SWOG/ECOG Randomized study of patients with GTR of pancreatic cancer treated with weekly gemcitabine or ci-5FU for 3 weeks before and 12 after concurrent chemo/RT (Ragine, 2008, JAMA) This study enrolled 451 patients with R0/R1 resection of pancreatic cancer. They were randomized to 3 weeks of either gemcitabine or ci-5FU → 5FU+RT (50.4 Gy @ 1.8 Gy/fraction) → 12 weeks of the original chemotherapy.
- Enrolled 451 with gross total resection
- Randomized: gemcitabine weekly or ci-5FU for 3 weekts
- All received 50.4 Gy radiation with 5FU → the original chemotherapy arm for 12 additional weeks.
- Results: Patterns of failure were the same in both arms:
- Distant metastastases: 71-77%
- local failure: 23-28%
- regional lymph nodes 7 - 8 %
- Trend toward improved median survival at 20.5 months from 16.9 months and OS3 at 31% from 22% (p=0.09)
Neoadjuvant Treatment
There are no completed phase III trials for neoadjuvant treatment.
Stessin reanalyzed the SEER database in 2008 and found 70 patients with pre-operative RT, 1478 patients with post-operative RT and 2337 patients treated with surgery alone. Median survival was 23 months in pre-op RT cohort, 17 months in the post-op RT cohort and 12 months in the surgery alone cohort.
Evans reported in 2008 a Phase II trial of 86 patients randomized to Chemo-RT with radiation given at 30 Gy at 3 Gy/fraction with concurrent chemotherapy of weekly gemcitabine for seven weeks followed by surgery. Radiation Therapy fields included the pancreaticoduodenal, portahepatic, superior mesenteric and celica axis lymph nodes. All patients were re-staged after chemo-radiation therapy. 85% went on to surgery. Median survival was 22.7 months, improved from 7 months for surgery alone and OS5 was 27%. For patients who received surgery median survival was 34% compared with 7 months for unresectable patients.
Unresectable Pancreatic Adenocarcinoma
There are a number of studies examining the treatment of unresectable pancreatic adenocarcinomas. The sentinel study was the GITSG 9273 study (Moertel) published in 1981. This study demonstrated a definitive role in chemo-radiation therapy as definitive treatment in unresectable cases. The FFCD/SFRO French study used non-standard chemo-radiation therapy and reported that CRT was more toxic and had worse survival outcomes. The non-standard regimen was very poorly tolerated.
GITSG 9273 Compared split course radiation therapy (40 Gy) with bolus 5FU to split course RT (60 Gy) with bolus 5FU to RT alone to 60 Gy. This study compared radiation alone, radiation with chemotherapy and dose escalated radiation therapy with chemotherapy. Both concommitant chemotherapy arms prolonged median survival compared with radiation alone.
- Enrolled 194 patients with unresectable pancreatic cancer
- Randomized to 3 arms:
- Split course EBRT alone to 60 Gy
- Split course EBRT to 40 Gy with concurrent bolus 5FU
- Split course EBRT to 60 Gy with concurrent bolus 5FU
- All arms received maintenance 5FU chemotherapy
- Both concommitant arms increased median survival over radiation therapy alone.
- Results:
- Median survival favored chemotherapy/radiotherapy arms with median survival 5.3 months (60 Gy alone, 9.7 months (40 Gy + 5FU), 9.6 months (60 Gy + 5FU).
- OS1 favored chemo/RT arms: 10% (RT alone); 35% (40 Gy + 5FU); 46% (60 Gy + 5FU)
- There were no statistically significant differences between the two chemotherapy/RT arms.
GERCOR Phase II/III Impact on chemo-radiotherapy with chemotherapy in locally advanced pancreatic cancer (Hugeut, 2007, JCO) This study examined 181 patients with locally advanced pancreatic cancer (unresectable) treated initially with 5FU or gemcitabine who had no evidence of progression after 3 months and then received additional chemotherapy or chemo-radiotherapy (physician's choice). Chemo-radiotherapy improved median progression free survival from 7.4 months to 10.8 months and overall survival from 11.7 months to 15 months.
FFCD/SFRO (French) Study 2 Arm trial of CRT (RT+5FU+CDDP followed by maintenance gemcitabine) compared to gemcitabine alone Cahuffert 2008, Ann Oncology.. This French study compared chemo-radiotherapy against gemcitabine chemotherapy alone. Induction chemo-radiotherapy was more toxic and had worse survival outcomes.
- Compared initial chemo-radiotherapy to chemotherapy alone
- Randomized: (1) RT to 60 Gy + ci5FU + CDDP → maintenance gemcitabine or (2) induction gemcitabine → maintenance gemcitabine.
- Median survival was worse in CRT arm at 8.6 months v. 13 months
- Overall survival was worse at 1 year in the CRT arm at 32% compared with 53% in the gemcitabine alone arm
- This study has been criticized for having a poor tolerance and using a non-standard chemo-RT regimen.
ECOG 4201 Compared Gemcitabine to gemcitabine + radiation (ASCO 2008). This study closed early due to slow accrual. It compared RT + gemcitabine to gemcitabine alone. 71 patients were accrued, Chemo-radiotherapy arm did better in median survival as well as overall survival at 2 years.
- Compared gemcitabine alone to gemcitabine + RT
- Closed early due to slow accrual
- Arm I: gemcitabine alone; Arm II: gemcitabine + RT.
- Median survival improved in RT+gemcitabine arm to 11 months from 9.2 months
- Overall survival at 2 year improved in RT_gemcitabine arm to 12% from 4%
Treatment Recommendations
At present there is controversy and various studies have come to differing conclusions and re-analysis of those same studies have come to different conclusions from the original author conclusions.
For resectable disease, R0 resection is indicated, followed by chemotherapy. The use of chemo-radiotherapy is controversial with the GITSG study recommending CRT, the ESPAC-1 (flawed by failure to standardize treatment, failure to complete or initiate planned treatments) failed to show an advantage. EORTC 20891 did, on reanalysis show an advantage to CRT.
Presently, for borderline resectable disease, there is no standard neoadjuvant treatment for pancreatic adenocarcinoma. There is an ongoing University of Michigan trial of neoadjuvant gemcitabine/Radiation, a local protocol at Dartmouth for induction docetaxel/gemcitabine → gemcitabine/RT and an MDACC protocol of gemcitabine+cisplatin → gemcitabine/RT for resectable disease. Radiation therapy is given to 45 - 50.4 Gy in 1.8 - 2 Gy fractions or 30 Gy in 3 Gy fractions. The current treatment paradigms are staging laparoscopy, stent placemnt if jaundice, and neoadjvant chemo/radiation → resection.
Presently for locally advanced pancreatic cancer in the US, the Standard definitive treatment recommendations are for unresectable pancreatic cancer are: CRT continuous infusion 5FU with Radiation therapy to 50 - 60 Gy in 1.8 - 2 Gy fractions or 30 Gy in 3 Gy fractions.
Metastatic Disease, Metastatic disease is treated with gemcitabine alone, gemcitabine with erlotinib or on a clinical trial.
NCIC Study of gemcitabine ± erlotinib (Moore 2007 JCO) demonstrated improved median survival of 5.9 months from 6.2 months and OS1 to 23% from 17%.There may be a role for bevacizumab as well (AViTA Trial).
AViTA Trial compared the addition of bevacizumab (Avastin) to gemcitabine/erlotinib (2008 ASCO). There was better PFS at 4.6 months from 3.6 months but no overall survival difference at 7.1 months and 6 months (not statistically significant) with the addition of avastin.
Radiation Fields and Techniques
Pancreatic cancer was traditionally treated with fairly large fields bearing in mind certain landmarks:
- Pancreas: L1-L2 vertebral bodies
- Celiac Axis: T12 vertebral body
- Superior Messenteric Artery: L1
The classic adjuvant radiation fields for pancreatic head disease cover the tumor bed, the pancreaticoduodenal nodes, local suprapancreatic nodes (but not the entire pancreas), celiac nodes, porta hepatis nodes, and the SMA/SMV nodes. For neoadjuvant and unresectable tumors the present trend is toward smaller fields. In the absence of CT/imaging guided planning, the following classical borders are used:
- AP:
- Superior: T10-T11
- Inferior: L3-L4
- Left: 2 cm from the left border of the vertebral body
- Right: 2 cm to the right of hte pre-op duodenum.
- Lateral
- Superior: match AP T10-T11
- Inferior: match AP L3-L4
- Posterior: 2 cm into the vertebral body
- Anterior: 2 cm anterior to gross preoperative disease or the duodenum
Stereotactic Body Radiotherapy has been the topic of several emerging studies.SBRT in unresectable pancreatic cancer may have promise for local control, but there has been significant duodenal toxicity. In addtion, RTOG 9704 demonstrated the general patterns of failure are distant metastases, in 71+% of cases, followed by a 23-25% local control failure.
Dose escalation trials using IMRT have been performed (Spalding 2007), increasing does from 52-66 Gy and achieving a boost dose of up to 85 Gy. This is technically possible as are the SBRT studies with cyberknife type technology, but again the distant disease is the primary mechanism of failure. There can be no durable control of distant disease without local control, but at present according to RTOG 9702 distant spread is the main failure rate in resected disease. Thus, improved local control, especially with added toxicity may not be an improvment.
Toxicity and Normal Tissue Tolerances
Liver < 30 Gy to ≤ 50%, Kidneys: ≥ 2/3 of one kidney ≤ 18 Gy; Spinal Cord ≤ 45 Gy; stomach and small bowel, < 50 Gy to small volumes.